Four different organic compounds are separated by a fractionating column.
The table shows the boiling points of the compounds.
The diagram shows the position in the fractionating column where they are separated.
| compound | boiling point/°C |
|---|---|
| Q | 69 |
| R | 196 |
| S | 90 |
| T | 125 |
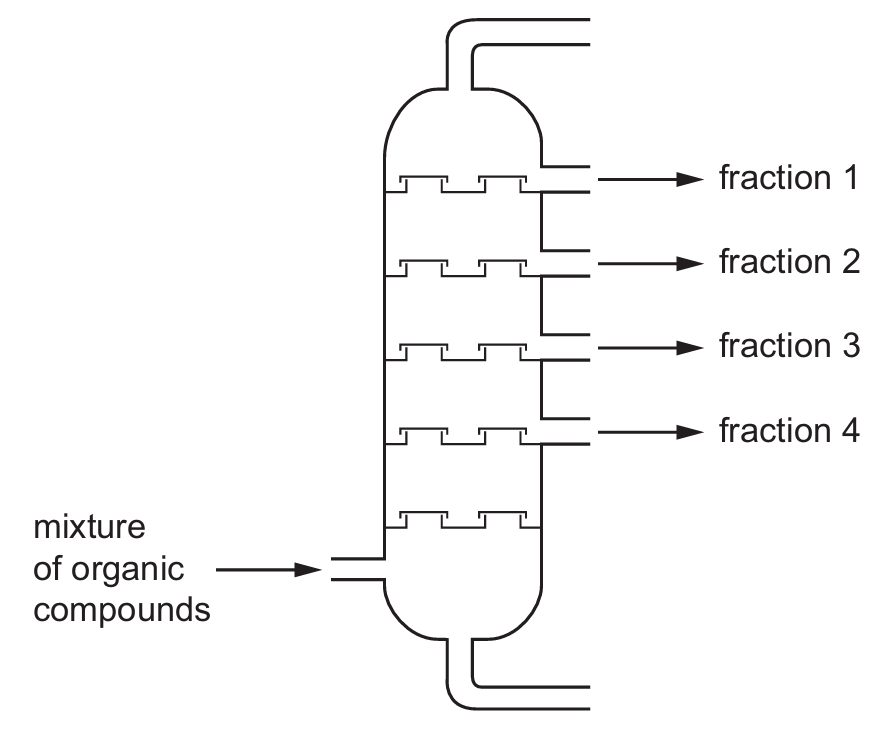
Which row identifies the compound in each fraction?
| fraction 1 | fraction 2 | fraction 3 | fraction 4 | |
|---|---|---|---|---|
| A | Q | S | T | R |
| B | Q | T | S | R |
| C | R | T | S | Q |
| D | R | S | T | Q |
▶️ Answer/Explanation
Ans: A
In fractional distillation, compounds with the lowest boiling points come off first (at the top of the column), while those with higher boiling points come off later (at the bottom).
Ordering the compounds by boiling point:
1. Q (69°C) – lowest boiling point, comes first
2. S (90°C)
3. T (125°C)
4. R (196°C) – highest boiling point, comes last
This matches option A where the fractions are ordered Q → S → T → R.
The diagram shows fraction 1 at the top (lowest boiling point) through to fraction 4 at the bottom (highest boiling point).
Sample M contains calcium carbonate and sodium nitrate.
The result of adding water to M, stirring and filtering is shown.
No chemical reaction occurs.
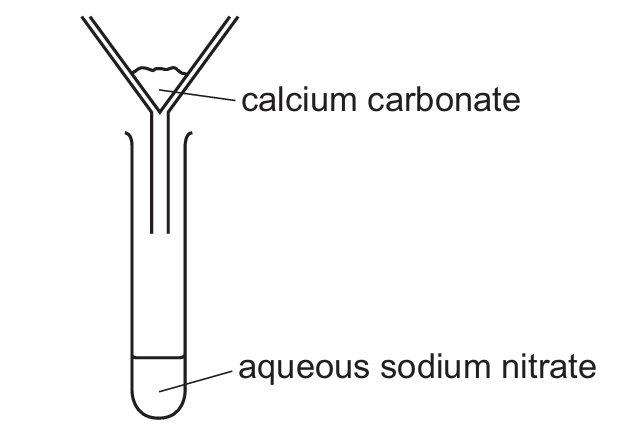
Which terms describe M, calcium carbonate and aqueous sodium nitrate?
| sample M | calcium carbonate | aqueous sodium nitrate | |
|---|---|---|---|
| A | compound | filtrate | residue |
| B | compound | residue | filtrate |
| C | mixture | filtrate | residue |
| D | mixture | residue | filtrate |
▶️ Answer/Explanation
Ans: D
Let’s analyze each component:
1. Sample M: Since it contains two different substances (calcium carbonate and sodium nitrate) physically mixed together, it’s a mixture, not a compound.
2. Calcium carbonate: Insoluble in water, so it remains as the solid residue after filtration.
3. Aqueous sodium nitrate: Soluble in water, so it passes through the filter paper to become the filtrate.
This matches option D where:
– M is a mixture
– Calcium carbonate is the residue
– Aqueous sodium nitrate is the filtrate
The key points are that no chemical reaction occurred (just physical separation) and calcium carbonate is insoluble while sodium nitrate is soluble.
A mixture containing an aqueous salt, sand and hot water is stirred.
The mixture is then poured into the apparatus shown.
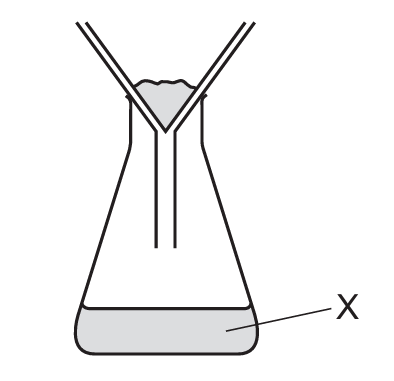
What is X?
A) a filtrate only
B) a residue only
C) a solute only
D) a solvent only
▶️ Answer/Explanation
Ans: A
The described process is filtration. When the mixture is poured into the filtration apparatus, the sand (insoluble solid) will be trapped by the filter paper as the residue, while the aqueous salt solution (filtrate) passes through. X refers to what comes through the filter paper, which is the filtrate – the liquid containing the dissolved salt. It’s not just the solvent (water) because it contains the dissolved salt, and it’s not the solute only because that would imply pure salt without water. The residue (sand) remains in the filter paper.
Question
Molten iron from the blast furnace contains impurities.
The process of turning the impure iron into steel involves blowing oxygen into the molten iron and adding calcium oxide.
What are the reasons for blowing in oxygen and adding calcium oxide?

▶️Answer/Explanation
Ans:A
