Hydrogen-oxygen fuel cells can be used to power cars.
Which processes produce the fuel of a hydrogen-oxygen fuel cell?
- the cracking of hydrocarbons
- the electrolysis of dilute sulfuric acid
- photosynthesis
- the electrolysis of molten aluminium oxide
A) 1 and 2
B) 1 and 4
C) 2 and 3
D) 3 and 4
▶️ Answer/Explanation
Ans: A
The fuel for hydrogen-oxygen fuel cells is hydrogen gas. Let’s evaluate each process:
1. Cracking of hydrocarbons can produce hydrogen as one of the products.
2. Electrolysis of dilute sulfuric acid produces hydrogen at the cathode.
3. Photosynthesis produces oxygen and glucose, not hydrogen.
4. Electrolysis of molten aluminium oxide produces aluminium and oxygen, not hydrogen.
Therefore, only processes 1 and 2 can produce hydrogen fuel.
Question
In separate experiments, electricity was passed through concentrated aqueous sodium chloride
and molten lead(II) bromide.
What would happen in both experiments?
A A halogen would be formed at the anode.
B A metal would be formed at the cathode.
C Hydrogen would be formed at the anode.
D Hydrogen would be formed at the cathode.
▶️Answer/Explanation
Ans: A
In the electrolysis of molten PbBr2, using graphite electrodes, the products formed are elemental lead at the negative electrode (cathode) and bromine gas at the positive electrode (anode). Lead ions are reduced by gaining one electron at the cathode, resulting in the formation of elemental lead. Bromide ions are oxidized to bromine gas by losing two electrons. This oxidation reaction occurs at the anode.
In the electrolysis of concentrated aqueous sodium chloride using graphite electrodes, the products formed are hydrogen gas at the negative electrode (cathode) and chlorine gas at the positive electrode (anode). Water molecules are reduced to hydrogen gas and hydroxide ions by gaining two electrons. This reduction reaction occurs at the cathode. Chloride ions are oxidized to chlorine gas by losing two electrons. This oxidation reaction occurs at the anode.
Question
Which gas is used as a fuel?
A helium
B hydrogen
C nitrogen
D oxygen
▶️Answer/Explanation
Ans: B
Hydrogen gas is used as fuel. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water.
Question
Hydrogen peroxide solution decomposes very slowly at room temperature to produce oxygen gas. This gas forms a rising foam when liquid detergent is added.
Five test-tubes are half filled with hydrogen peroxide solution. A drop of liquid detergent is added to each one. Different metal oxides are added to four of the test-tubes and the height of the foam formed after 1 minute is measured. The results are shown.
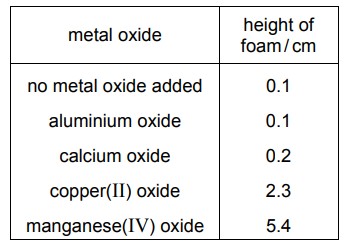
Which conclusion can be drawn from these results?
A. Metal oxides do not affect the rate of this reaction.
B. All metal oxides increase the rate of this reaction and act as catalysts.
C. Manganese(IV) oxide is the best catalyst of the four metal oxides tested.
D. Only transition element oxides increase the rate of this reaction.
▶️Answer/Explanation
Ans:
C
Aluminium oxide does not affect the rate of this reaction because the height of foam does not change after its addition.
Manganese(IV) oxide is the best catalyst of the four metal oxides tested since it gave the maximum height of form formed.
