Which process involves a chemical change?
A) adding sodium chloride to water
B) adding magnesium to hydrochloric acid
C) heating solid iodine until it turns into a gas
D) melting lead
▶️ Answer/Explanation
Ans: B
A chemical change involves the formation of new substances. Let’s analyze each option:
A) Dissolving NaCl in water is a physical change – the NaCl dissociates into ions but can be recovered by evaporation.
B) Magnesium reacting with hydrochloric acid produces magnesium chloride and hydrogen gas – a clear chemical change with new substances formed.
C) Iodine subliming is a physical change of state (solid to gas) with no new substances formed.
D) Melting lead is a physical change (change of state from solid to liquid).
Only option B represents a chemical change where new substances are formed.
Which process is a chemical change?
A) boiling water
B) cooking an egg
C) dissolving sugar
D) melting ice cubes
▶️ Answer/Explanation
Ans: B
A chemical change results in the formation of new substances with different chemical properties. Cooking an egg involves the denaturation of proteins (irreversible change), which is a chemical change. Boiling water (A) and melting ice (D) are both physical changes as they only change the state of matter. Dissolving sugar (C) is also a physical change because the sugar molecules remain unchanged and can be recovered by evaporation.
Question
A sequence of changes involving sulfur is shown.
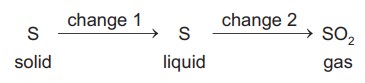
Which row describes the changes?
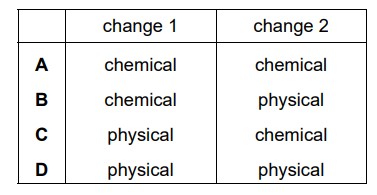
▶️Answer/Explanation
Ans:
C
The change from solid sulfur to liquid sulfur is a physical change, specifically a change of state or phase change.
The change from liquid sulfur to sulfur dioxide gas (SO2) is a chemical change. During this process, the chemical composition of the substance changes as sulfur undergoes a chemical reaction to form sulfur dioxide.
Question
Which process involves a physical change?
A heating calcium carbonate
B burning wood
C melting an ice cube
D mixing an acid and a base
▶️Answer/Explanation
Ans:C
The melting of an ice cube represents a physical change, specifically a phase change, as it involves the transformation of a solid (ice) into a liquid (water) without any alteration in chemical composition.
