A student reacts strips of zinc with dilute sulfuric acid and measures the time taken to produce 100 cm3 of hydrogen.
The experiment is repeated using different conditions.
The results are shown in the table.
| experiment | time to produce 100 cm3 of hydrogen/s |
|---|---|
| 1 | 250 |
| 2 | 100 |
Which changes in conditions produce the results shown in experiment 2?
- Add a catalyst.
- Dilute the acid.
- Use zinc powder.
- Heat the acid.
A) 1, 3 and 4
B) 1 and 4 only
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: A
To understand why option A is correct, let’s analyze each condition:
1. Adding a catalyst increases the reaction rate by providing an alternative pathway with lower activation energy.
2. Diluting the acid would actually slow down the reaction (decrease rate), so this can’t be correct.
3. Using zinc powder increases the surface area, which increases the reaction rate.
4. Heating the acid provides more energy to the particles, increasing their collision frequency and energy.
Since experiment 2 shows a faster reaction (100s vs 250s), we need conditions that increase the rate. Only options 1, 3, and 4 would increase the rate, making A the correct answer.
Reaction 1 and reaction 2 both produce a gas. The total volume of gas produced in each reaction is measured every minute for 10 minutes.
A graph of the results is shown.
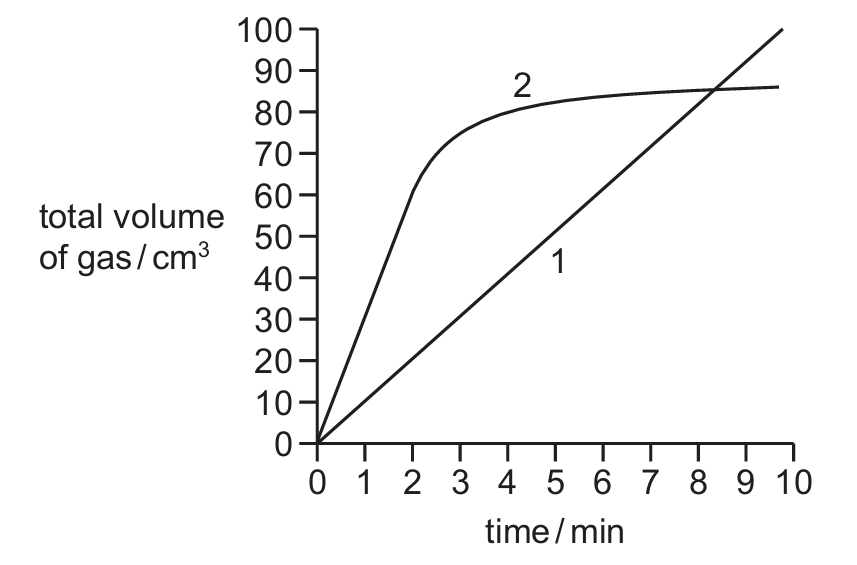
Which row describes how the rate of reaction changes, if at all, during each reaction?
| reaction 1 | reaction 2 | |
|---|---|---|
| A | the rate is constant | the rate decreases after 2 minutes |
| B | the rate increases | the rate increases |
| C | the rate increases | the rate decreases after 2 minutes |
| D | the rate is constant | the rate increases |
▶️ Answer/Explanation
Ans: A
From the graph description:
Reaction 1: The straight line indicates a constant rate of reaction (volume increases steadily with time).
Reaction 2: The steep initial slope followed by leveling off indicates the rate decreases after 2 minutes (likely because reactants are being used up).
Therefore, option A correctly describes both reactions – Reaction 1 has a constant rate while Reaction 2’s rate decreases after 2 minutes.
Which two pieces of apparatus are most useful to measure the rate of a reaction in which a gas is given off?
A) accurate balance and gas syringe
B) accurate balance and thermometer
C) gas syringe and stop-watch
D) stop-watch and thermometer
▶️ Answer/Explanation
Ans: C
To measure the rate of a gas-producing reaction, we need to measure either:
- The volume of gas produced over time (using a gas syringe)
- The time taken to produce a certain amount of gas (using a stop-watch)
Option C combines both these essential pieces of apparatus. A gas syringe measures the volume of gas produced, while a stop-watch measures the time taken. The other options either include unnecessary equipment (thermometer) or less direct measurement methods (balance measures mass change, which isn’t as straightforward for gas evolution).
Four students collect the gas produced from the reaction of calcium carbonate with dilute hydrochloric acid. Each student records the time taken to collect a volume of gas.
Which results show the highest average rate of reaction?
A) 15 cm3 of gas collected in 20 seconds
B) 50 cm3 of gas collected in 40 seconds
C) 75 cm3 of gas collected in 80 seconds
D) 90 cm3 of gas collected in 100 seconds
▶️ Answer/Explanation
Ans: B
To determine the highest average rate of reaction, we calculate the rate of gas production (volume/time) for each option:
A) 15 cm3/20 s = 0.75 cm3/s
B) 50 cm3/40 s = 1.25 cm3/s
C) 75 cm3/80 s ≈ 0.94 cm3/s
D) 90 cm3/100 s = 0.90 cm3/s
Option B has the highest rate at 1.25 cm3/s. Even though option D produces more total gas, it takes proportionally longer, resulting in a lower average rate.
This shows that the highest rate isn’t necessarily the one with the most product, but rather the one that produces gas fastest relative to the time taken.
