Limonene is a volatile liquid which smells of oranges.
(a) A teacher placed a beaker of limonene at the front of a classroom.
At first, the students at the back of the classroom could not smell the limonene.
After two minutes, the smell of limonene had spread throughout the classroom.
The air in the classroom was still and calm.
(i) Explain these observations using the kinetic particle model. [3]
(ii) The melting point of limonene is –74°C.
The boiling point of limonene is 176°C.
What is the physical state of limonene at –80°C?
Explain your answer.[2]
(b) An enzyme present in peppermint plants is a catalyst for the oxidation of limonene.
State what is meant by the terms:
(i) catalyst [1]
(ii) oxidation [1]
(c) Limonene can be made from a colourless compound called α-terpineol.
The structure of α-terpineol is shown.
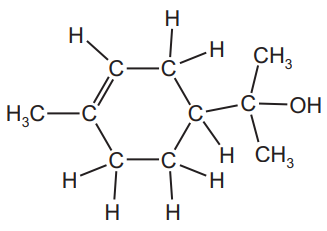
(i) What feature of the structure of the α-terpineol molecule shows that it is an unsaturated compound?[1]
(ii) Describe how the colour of aqueous bromine changes when an excess of α-terpineol is added to it.
from ___ to ___ [2] [Total: 10]
▶️ Answer/Explanation
(a)(i)
Limonene particles evaporate from the liquid state into the air (1). These gas particles then diffuse throughout the classroom (1), moving randomly from areas of high concentration (near the beaker) to low concentration (back of class) until evenly distributed (1).
(a)(ii) Ans: solid
At –80°C (which is below its melting point of –74°C), limonene exists as a solid because the temperature isn’t high enough to overcome the intermolecular forces holding the particles in a fixed position (1). The particles only gain sufficient energy to move freely when heated above –74°C (1).
(b)(i) Ans: A substance that increases reaction rate without being consumed
The enzyme speeds up the oxidation of limonene while remaining unchanged itself.
(b)(ii) Ans: Loss of electrons/gain of oxygen
Oxidation refers to either the addition of oxygen to a substance or the removal of electrons from it.
(c)(i) Ans: C=C double bond
The presence of a carbon-carbon double bond in the structure indicates unsaturation, meaning it can undergo addition reactions.
(c)(ii) Ans: orange/brown to colourless
Bromine water (orange) decolorizes when it reacts with the double bond in α-terpineol through an addition reaction (1). The colour change confirms the compound’s unsaturation (1).
