(a) Fig. 4.1 shows the displayed formula of compound A.
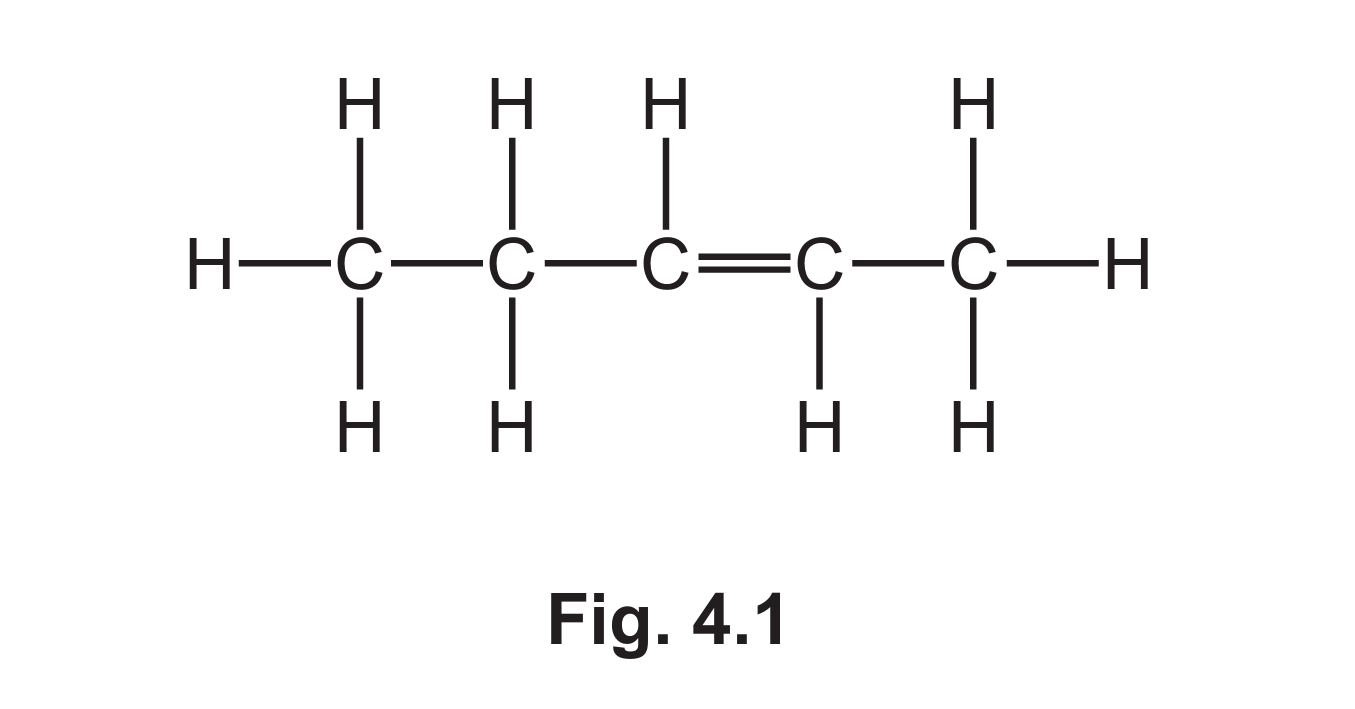
(i) Explain why compound A is described as unsaturated.
(ii) Explain why compound A is a hydrocarbon.
(iii) Deduce the molecular formula of compound A.
(b) Compound A reacts with steam to produce an alcohol.
(i) State the general formula for the homologous series of alcohols.
(ii) Ethanol is an alcohol which can be manufactured by fermentation.
– Name two substances needed for fermentation.
– Give two conditions needed for fermentation.
(iii) State one use of ethanol.
(c) A compound in the same homologous series as compound A reacts with ozone, \( O_3 \), to form compound B.
(i) Define the term homologous series.
(ii) The molecular formula for compound B is \( C_6H_{12}O_3 \).
Complete Table 4.1 to calculate the relative molecular mass of \( C_6H_{12}O_3 \).
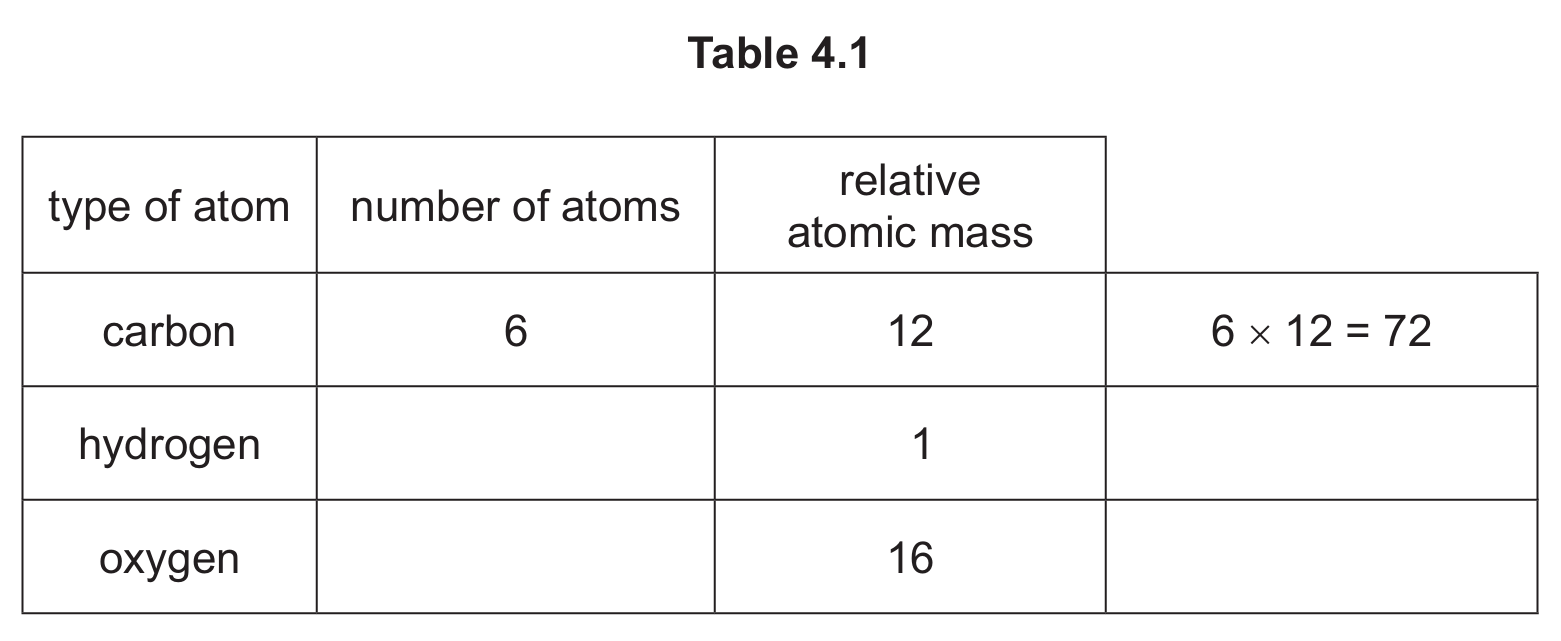
relative molecular mass = ………………………… [2]
▶️ Answer/Explanation
(a)(i) C=C bond / carbon-carbon double bond
Detailed explanation: An unsaturated compound contains at least one double or triple bond between carbon atoms. The presence of a C=C double bond (as shown in Fig. 4.1) means the compound is unsaturated because it can undergo addition reactions where atoms add across the double bond.
(a)(ii) contains carbon and hydrogen only / contains carbon and hydrogen and no other element
Detailed explanation: A hydrocarbon is defined as any compound that consists exclusively of carbon and hydrogen atoms. Since compound A only contains these two elements (as evident from its displayed formula), it qualifies as a hydrocarbon.
(a)(iii) \( C_6H_{10} \)
Detailed explanation: By counting the atoms in the displayed formula (Fig. 4.1), we can determine the molecular formula. The structure shows 6 carbon atoms and 10 hydrogen atoms, giving the molecular formula \( C_6H_{10} \).
(b)(i) \( C_nH_{2n+1}OH \)
Detailed explanation: The general formula for alcohols is \( C_nH_{2n+1}OH \), where n represents the number of carbon atoms. This formula accounts for the hydroxyl (-OH) functional group characteristic of alcohols.
(b)(ii) substances needed: (aqueous) glucose and yeast
conditions: any two of: absence of oxygen, 25-45°C, aqueous/in water
Detailed explanation: Fermentation requires glucose as the substrate and yeast as the microorganism that carries out the process. The optimal conditions include: anaerobic conditions (absence of oxygen), warm temperatures (25-45°C) that allow yeast enzymes to function efficiently, and an aqueous environment for the reaction to occur.
(b)(iii) solvent / fuel
Detailed explanation: Ethanol has multiple uses including as a solvent in various industries (perfumes, medicines) and as a biofuel or fuel additive due to its combustible properties.
(c)(i) family of similar chemical compounds / compounds with similar chemical properties with same functional group
Detailed explanation: A homologous series is a group of organic compounds that share: 1) the same general formula, 2) similar chemical properties, and 3) a gradual change in physical properties. Members differ by a \( CH_2 \) unit and have the same functional group.
(c)(ii) 132
Detailed explanation: To calculate the relative molecular mass of \( C_6H_{12}O_3 \):
Carbon: 6 atoms × 12 = 72
Hydrogen: 12 atoms × 1 = 12
Oxygen: 3 atoms × 16 = 48
Total = 72 + 12 + 48 = 132
The completed table would show 12 hydrogen atoms and 3 oxygen atoms.
Coal gas is made by heating coal in the absence of air. The list shows the main gases present in coal gas.
- carbon dioxide
- carbon monoxide
- ethene
- hydrogen
- methane
- nitrogen
(a)(i) Which one of these gases is an alkane
(a)(ii) Draw the structure of a molecule of ethene. Show all of the atoms and all of the bonds.
(a)(iii) Describe how aqueous bromine can be used to tell the difference between methane and ethene
(b) Ethene molecules react with each other to form poly(ethene).
(b)(i) What is the name given to this type of chemical reaction?
(b)(ii) Which one of the following words describes the ethene molecules in this reaction?
Draw a circle around the correct answer.
elements mixtures monomers polymers
(b)(iii) Poly(ethene) is a non-biodegradable plastic.
What is meant by the term non-biodegradable?
(b)(iv) Describe one pollution problem caused by non-biodegradable plastics
(c) Ethanol can be made from ethene and one other reactant.
● Name the other reactant
● State the conditions needed to make ethanol from ethene
▶️ Answer/Explanation
(a)(i) Ans: Methane
Methane (\( \text{CH}_4 \)) is the only alkane in the list, as alkanes are saturated hydrocarbons with single bonds.
(a)(ii) Ans: 
Ethene (\( \text{C}_2\text{H}_4 \)) has a double bond between the two carbon atoms, with each carbon bonded to two hydrogen atoms.
(a)(iii) Ans: Aqueous bromine remains orange with methane (no reaction) but decolorizes with ethene (addition reaction).
Ethene reacts with bromine, breaking the double bond and forming 1,2-dibromoethane, while methane (saturated) does not react.
(b)(i) Ans: Polymerization (or addition polymerization).
Ethene monomers undergo addition polymerization to form the polymer poly(ethene).
(b)(ii) Ans: Monomers
Ethene molecules are monomers, the small units that join to form the polymer poly(ethene).
(b)(iii) Ans: Cannot be broken down by natural organisms (e.g., bacteria/fungi).
Non-biodegradable materials persist in the environment due to their resistance to microbial decomposition.
(b)(iv) Ans: Causes harm to wildlife (e.g., ingestion by animals, blockages in drains).
Plastic waste accumulates in ecosystems, posing physical hazards to animals and clogging waterways.
(c) Ans: Other reactant: Steam (\( \text{H}_2\text{O} \)). Conditions: High temperature (~300°C), phosphoric acid catalyst.
Ethene reacts with steam under these conditions to form ethanol: \( \text{C}_2\text{H}_4 + \text{H}_2\text{O} \rightarrow \text{C}_2\text{H}_5\text{OH} \).
