(a) Fig. 4.1 shows the displayed formula of compound A.
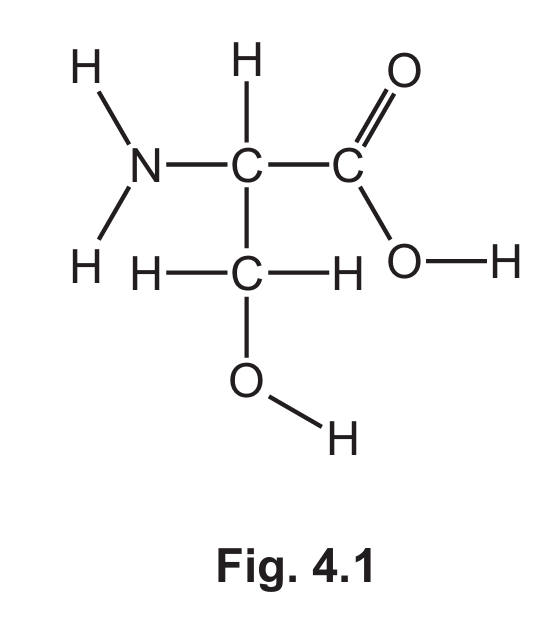
(i) On Fig 4.1 draw a circle around the carboxylic acid functional group.
(ii) Deduce the molecular formula of compound A.
(b) Compound A reacts with ethanol to produce a compound with the molecular formula \( C_5H_{11}NO_3 \).
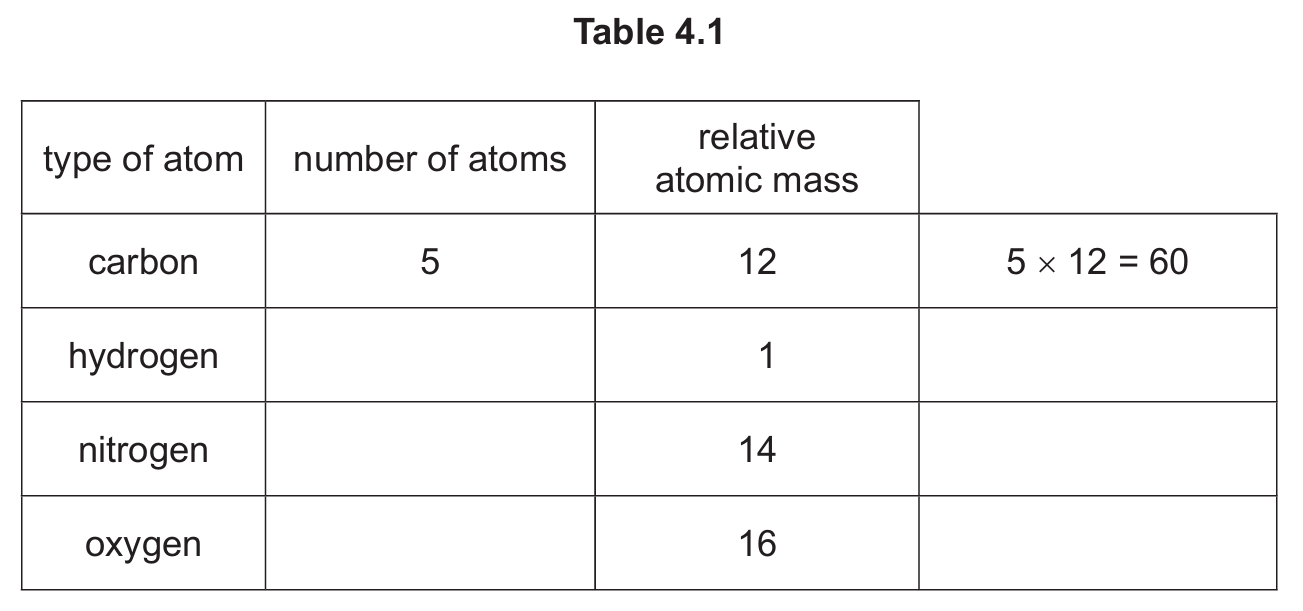
Complete Table 4.1 to calculate the relative molecular mass of \( C_5H_{11}NO_3 \).
(c) Table 4.2 shows the names, formulae and boiling points of methanol, ethanol, propanol and butanol.
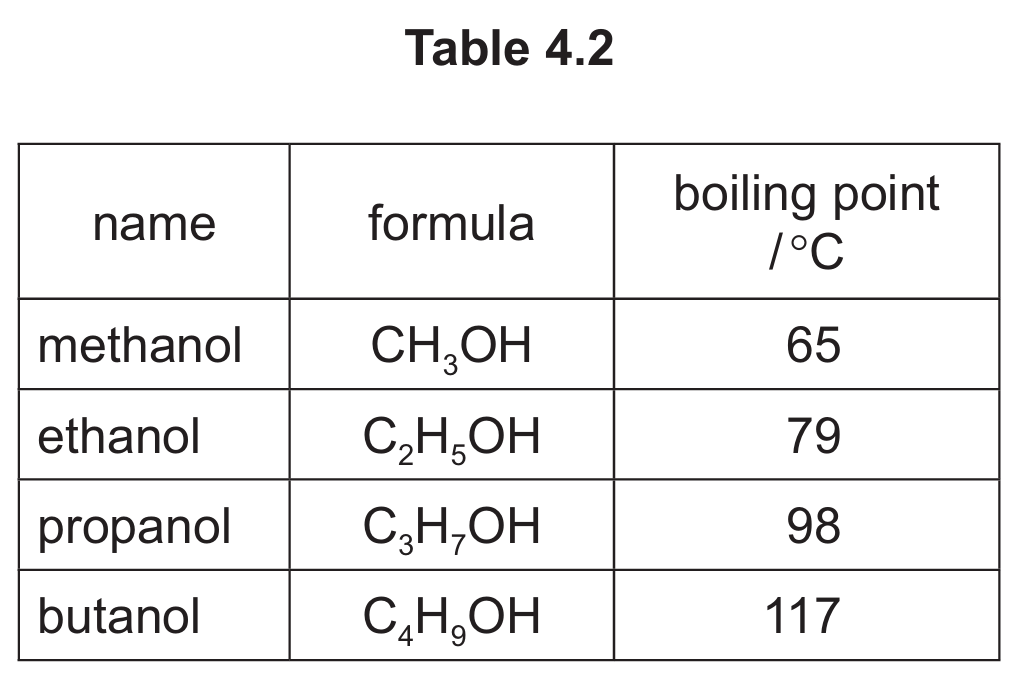
Use the information in Table 4.2 to answer these questions.
(i) Name the homologous series that includes methanol, ethanol, propanol and butanol.
(ii) Deduce the general formula of this homologous series.
(iii) State the trend in the boiling point of this homologous series as the number of carbon atoms increases.
(d) Ethanol can be manufactured by an addition reaction.
(i) Name two substances and state two conditions required.
(ii) Draw the displayed formula of ethanol.
(iii) Name the toxic gas produced when ethanol undergoes incomplete combustion.
▶️ Answer/Explanation
(a)(i) COOH group circled
The carboxylic acid functional group is -COOH. In the displayed formula, this group should be clearly identified and circled.
(a)(ii) \( C_3H_7NO_3 \)
By counting all atoms in the displayed formula of compound A, we find it contains 3 carbon atoms, 7 hydrogen atoms, 1 nitrogen atom, and 3 oxygen atoms.
(b) 133
Calculation:
Carbon: 5 × 12 = 60
Hydrogen: 11 × 1 = 11
Nitrogen: 1 × 14 = 14
Oxygen: 3 × 16 = 48
Total = 60 + 11 + 14 + 48 = 133
(c)(i) alcohol(s)
All these compounds contain the -OH functional group, which defines the alcohol homologous series.
(c)(ii) \( C_nH_{2n+1}OH \)
This is the general formula for alcohols, where n represents the number of carbon atoms.
(c)(iii) Increases
As the number of carbon atoms increases, the boiling point increases due to stronger van der Waals forces between larger molecules.
(d)(i)
Substances required:
• ethene
• steam/water
Conditions:
• 300°C temperature
• 6000 kPa/60 atm pressure
• phosphoric acid catalyst
(d)(ii) 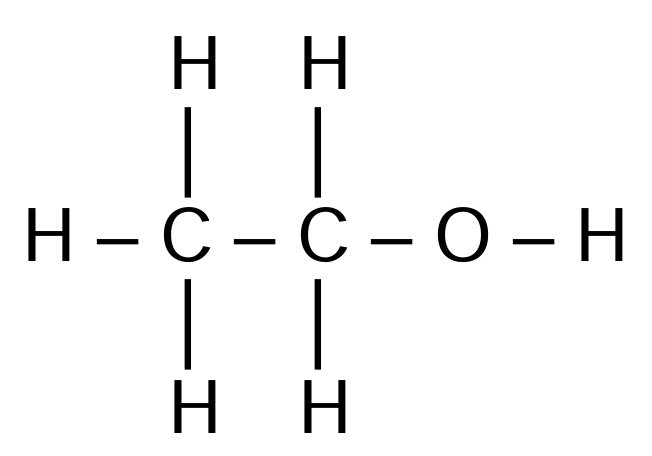
(d)(iii) carbon monoxide
Incomplete combustion of ethanol occurs when there’s insufficient oxygen, producing toxic carbon monoxide gas instead of carbon dioxide.
Coal gas is made by heating coal in the absence of air. The list shows the main gases present in coal gas.
- carbon dioxide
- carbon monoxide
- ethene
- hydrogen
- methane
- nitrogen
(a)(i) Which one of these gases is an alkane
(a)(ii) Draw the structure of a molecule of ethene. Show all of the atoms and all of the bonds.
(a)(iii) Describe how aqueous bromine can be used to tell the difference between methane and ethene
(b) Ethene molecules react with each other to form poly(ethene).
(b)(i) What is the name given to this type of chemical reaction?
(b)(ii) Which one of the following words describes the ethene molecules in this reaction?
Draw a circle around the correct answer.
elements mixtures monomers polymers
(b)(iii) Poly(ethene) is a non-biodegradable plastic.
What is meant by the term non-biodegradable
(b)(iv) Describe one pollution problem caused by non-biodegradable plastics
(c) Ethanol can be made from ethene and one other reactant.
● Name the other reactant
● State the conditions needed to make ethanol from ethene
▶️ Answer/Explanation
(a)(i) Ans: Methane
Methane (\( \text{CH}_4 \)) is the only alkane in the list, as alkanes are saturated hydrocarbons with single bonds.
(a)(ii) Ans: 
Ethene (\( \text{C}_2\text{H}_4 \)) has a double bond between the two carbon atoms, with each carbon bonded to two hydrogen atoms.
(a)(iii) Ans: Aqueous bromine remains orange with methane (no reaction) but decolorizes with ethene (addition reaction).
Ethene reacts with bromine, breaking the double bond and forming 1,2-dibromoethane, while methane (saturated) does not react.
(b)(i) Ans: Polymerization (or addition polymerization).
Ethene monomers undergo addition polymerization to form the polymer poly(ethene).
(b)(ii) Ans: Monomers
Ethene molecules are monomers, the small units that join to form the polymer poly(ethene).
(b)(iii) Ans: Cannot be broken down by natural organisms (e.g., bacteria/fungi).
Non-biodegradable materials persist in the environment due to their resistance to microbial decomposition.
(b)(iv) Ans: Causes harm to wildlife (e.g., ingestion by animals, blockages in drains).
Plastic waste accumulates in ecosystems, posing physical hazards to animals and clogging waterways.
(c) Ans: Other reactant: Steam (\( \text{H}_2\text{O} \)). Conditions: High temperature (~300°C), phosphoric acid catalyst.
Ethene reacts with steam under these conditions to form ethanol: \( \text{C}_2\text{H}_4 + \text{H}_2\text{O} \rightarrow \text{C}_2\text{H}_5\text{OH} \).
