The hydroxides of the Group I metals are soluble in water. Most other metal hydroxides are insoluble in water.
(a) (i) Crystals of lithium chloride can be prepared from lithium hydroxide by titration.
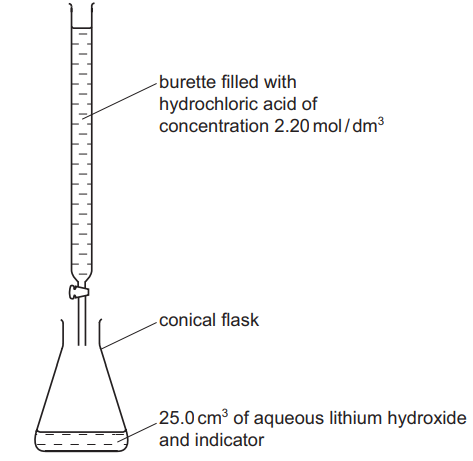
25.0 cm³ of aqueous lithium hydroxide is pipetted into the conical flask. A few drops of an indicator are added. Dilute hydrochloric acid is added slowly to the alkali until the indicator just changes colour. The volume of acid needed to neutralize the lithium hydroxide is noted.
A neutral solution of lithium chloride, which still contains the indicator, is left. Describe how you could obtain a neutral solution of lithium chloride which does not contain an indicator. [2]
(ii) You cannot prepare a neutral solution of magnesium chloride by the same method. Describe how you could prepare a neutral solution of magnesium chloride. [3]
(b) The concentration of the hydrochloric acid was 2.20 mol/dm³. The volume of acid needed to neutralize the 25.0 cm³ of lithium hydroxide was 20.0 cm³. Calculate the concentration of the aqueous lithium hydroxide. [2]
LiOH + HCl → LiCl + H₂O
(c) Lithium chloride forms three hydrates. They are LiCl·H₂O, LiCl·2H₂O and LiCl·3H₂O. Which one of these three hydrates contains 45.9% of water? Show how you arrived at your answer. [3] [Total: 10]
▶️ Answer/Explanation
(a)(i) Ans:
1. Add activated charcoal to adsorb the indicator (1). 2. Filter the solution to remove the charcoal with adsorbed indicator (1).
Alternative method: Repeat the titration without indicator, using the same volume of acid determined in the first titration (2).
(a)(ii) Ans:
1. React magnesium oxide/carbonate/hydroxide with hydrochloric acid until no more reacts (1). 2. Filter to remove excess solid (1). 3. The filtrate is a neutral solution of MgCl₂ (1).
(b) Ans: 1.76 mol/dm³
Moles of HCl = 2.20 × 0.020 = 0.044 mol (1).
Since 1:1 ratio, moles of LiOH = 0.044 mol.
Concentration = 0.044 ÷ 0.025 = 1.76 mol/dm³ (1).
(c) Ans: LiCl·2H₂O
1. Calculate molar mass of LiCl·2H₂O = 42.5 (LiCl) + 36 (2H₂O) = 78.5 g/mol (1).
2. % water = (36 ÷ 78.5) × 100 = 45.9% (1).
3. Other hydrates give different percentages (1).
