This question is about Group IV elements and their compounds.
(a) The changes of state of lead are shown.

Name the changes of state represented by A and B.[2]
A
B
(b) Use the kinetic particle model to describe the differences between liquid lead and lead gas in terms of:[4]
- the separation of the particles
- the motion of the particles.
(c) Lead is extracted from lead(II) oxide by heating with carbon.
PbO + C → Pb + CO
Describe how this equation shows that lead(II) oxide is reduced.[1]
(d) Lead is a pollutant of the air.
(i) State one source of lead in the air.[1]
(ii) State one adverse effect of lead on health.[1]
(e) Diamond is a form of carbon.
The structure of diamond is shown.

(i) Choose the word which best describes the structure of diamond.
Draw a circle around your chosen answer.[1]
giant ionic metallic simple
(ii) Name the type of bonding in diamond.[1]
(iii) Give one use of diamond.[1]
(iv) Deduce the electronic structure of carbon.
Use the Periodic Table to help you.[1] [Total: 13]
▶️ Answer/Explanation
(a) Ans: A: freezing, B: boiling
From the diagram: A shows liquid → solid (freezing), B shows liquid → gas (boiling).
(b) Ans:
Separation: Liquid particles are close but can move, gas particles are far apart.
Motion: Liquid particles slide past each other, gas particles move freely in all directions.
(c) Ans: Lead oxide loses oxygen
Reduction is gain of electrons/loss of oxygen. PbO loses O to form Pb.
(d)(i) Ans: Leaded gasoline/industrial emissions
Historically from leaded petrol, now mainly industrial processes.
(d)(ii) Ans: Neurotoxin affecting brain development
Lead accumulates in body, causing neurological damage.
(e)(i) Ans: giant
Diamond has a continuous 3D network of covalent bonds.
(e)(ii) Ans: covalent
Carbon atoms share electrons in strong covalent bonds.
(e)(iii) Ans: Cutting tools/drill bits
Uses diamond’s hardness (strong covalent structure).
(e)(iv) Ans: 2,4
Carbon (Group IV) has 4 valence electrons (2 in first shell, 4 in outer).
The diagram shows a blast furnace used in the extraction of iron.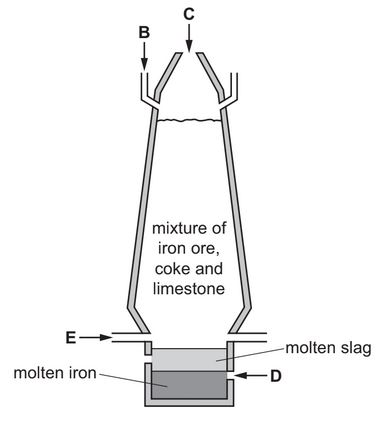
(a) Air is blown into the furnace.
State which letter on the diagram, B, C, D or E, shows where air is blown into the furnace.
(b)(i) Complete the chemical equation for the reduction of iron(III) oxide in the blast furnace.
\(Fe_2O_3 + 3C → ……Fe + ……CO\)
(b)(ii) Explain how this equation shows that iron(III) oxide is reduced.
(c) Calcium carbonate (limestone) is added to the blast furnace.
The calcium carbonate undergoes thermal decomposition.
(c)(i) Complete the word equation for this reaction.
(c)(ii) One of the products of this reaction reacts with impurities in the iron to form slag.
Use the information in the diagram to suggest how you know that molten slag is less dense than molten iron.
(d)(i) Use words from the list to complete these sentences about how steel is made from iron.
acidic basic chlorides methane neutral
nitrogen oxides oxygen sulfates
A gas is blown through the molten iron. The name of this gas is …………………… .
Acidic gases are formed. These acidic gases react with …………………… ……………………
(d)(ii) State one use of mild steel.
(d)(iii) Metals such as chromium are added to iron to make stainless steel.
The symbol for an isotope of chromium is shown.
\(_{24}^{53}Cr\)
Deduce the number of electrons, neutrons and protons in one atom of this isotope of chromium.
(e) Chromium conducts electricity and is shiny.
Give two other physical properties of chromium that are characteristic of all metals.
▶️ Answer/Explanation
(a) Ans: E
Air is blown into the furnace at point E (tuyeres), where it reacts with coke to produce carbon monoxide and heat.
(b)(i) Ans: \(Fe_2O_3 + 3C → 2Fe + 3CO\)
The balanced equation shows 2 iron atoms and 3 carbon monoxide molecules produced per formula unit of iron(III) oxide.
(b)(ii) Ans: Iron(III) oxide loses oxygen (gains electrons), forming iron.
Reduction involves the loss of oxygen. Here, \(Fe_2O_3\) loses oxygen atoms to form \(Fe\), while carbon is oxidized to \(CO\).
(c)(i) Ans: Calcium carbonate → calcium oxide + carbon dioxide
Thermal decomposition of limestone (\(CaCO_3\)) yields calcium oxide (\(CaO\)) and \(CO_2\).
(c)(ii) Ans: Slag floats above molten iron in the furnace.
Less dense materials float atop denser ones. The diagram shows slag layered above iron, indicating lower density.
(d)(i) Ans: Oxygen; basic oxides
Oxygen is blown to remove impurities (e.g., carbon) as acidic gases, which react with basic oxides (e.g., \(CaO\)) to form slag.
(d)(ii) Ans: Car bodies, machinery, or construction.
Mild steel’s ductility and strength make it ideal for structural applications.
(d)(iii) Ans: Electrons: 24; Neutrons: 29; Protons: 24
For \(_{24}^{53}Cr\): Atomic number (protons/electrons) = 24. Neutrons = Mass number (53) – protons (24) = 29.
(e) Ans: Malleable and ductile (or conducts heat).
Metals like chromium can be hammered into sheets (malleable) or drawn into wires (ductile) due to metallic bonding.
