This question is about sulfur, sulfur compounds and the water from a sulfur spring. A sulfur spring is a natural source of water containing sulfur.
(a) The table shows the mass of ions present in a 1000cm3 sample of water from a sulfur spring.
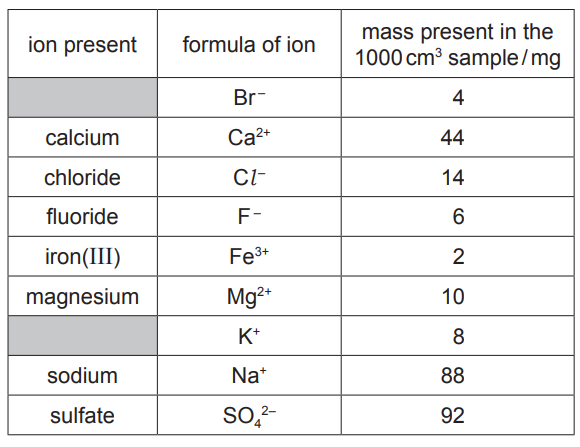
Answer these questions using only information from the table.
(i) Which negative ion is present in the lowest concentration?[1]
(ii) Give the name of the compound formed from only K+ and Br– ions.[1]
(iii) Calculate the mass of calcium ions present in a 250cm3 sample of this water.
mass of calcium ions = mg [1]
(iv) Complete the equation to show the formation of a fluoride ion from a fluorine atom.[1]
F + ____ → F–
(b) Describe a test for sulfate ions.[2]
test
observations
(c) Solid sulfur is found around the edge of sulfur springs.
(i) When heated, sulfur undergoes sublimation.
What is meant by the term sublimation?[1]
(ii) Sulfur reacts with hot concentrated sulfuric acid.
Complete the chemical equation for this reaction.
S + ___ H2SO4 → 2H2O + ___ SO2 [2]
(iii) The table shows the solubility of sulfur and zinc in an organic solvent and in water. The organic solvent boils at 46°C.
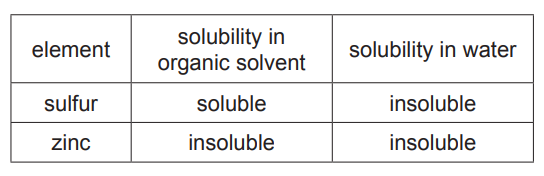
Use the information in the table to suggest how to obtain pure, dry samples of both sulfur and zinc from a mixture of sulfur powder and zinc powder.[4]
(d) The structure of a sulfur compound is shown.
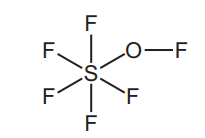
Deduce the molecular formula of this compound showing the number of sulfur, fluorine and oxygen atoms.[1][Total: 14]
▶️ Answer/Explanation
(a)(i) Ans: Br– / bromide
Bromide has the lowest mass (0.4 mg) in the table, indicating the lowest concentration.
(a)(ii) Ans: Potassium bromide
K+ and Br– combine to form the ionic compound potassium bromide (KBr).
(a)(iii) Ans: 11 mg
For 250 cm3, mass of Ca2+ = (44 mg ÷ 1000 cm3) × 250 cm3 = 11 mg.
(a)(iv) Ans: e–
A fluorine atom gains an electron to form a fluoride ion: F + e– → F–.
(b) Ans: Acidified barium chloride/nitrate test
Test: Add acidified BaCl2/Ba(NO3)2 to the solution.
Observation: White precipitate (BaSO4) confirms sulfate ions.
(c)(i) Ans: Sublimation
Sublimation is the direct transition from solid to gas without passing through the liquid phase.
(c)(ii) Ans: 2 H2SO4, 3 SO2
Balanced equation: S + 2H2SO4 → 2H2O + 3SO2.
(c)(iii) Ans: Separation steps
1. Dissolve the mixture in organic solvent (sulfur dissolves, zinc does not).
2. Filter to separate zinc (residue).
3. Evaporate the solvent from the filtrate to obtain sulfur.
4. Dry the zinc residue.
(d) Ans: SF6O
The structure shows 1 sulfur (S), 6 fluorine (F), and 1 oxygen (O) atom.
(a) The table shows the ions present in a \(1000~cm^3\) sample of blood plasma.
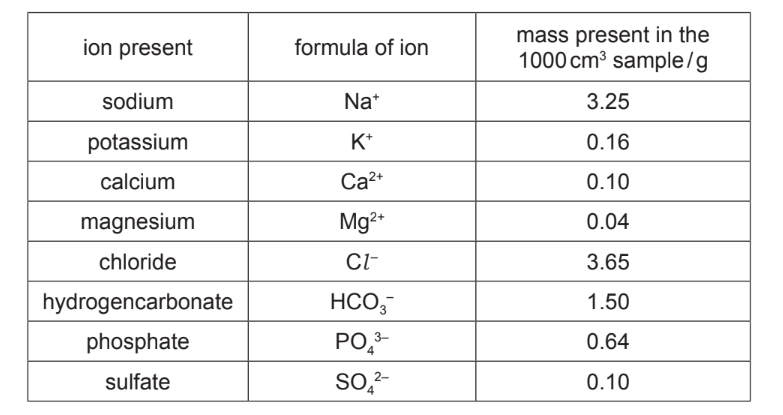
Answer these questions using only information from the table.
(i) Which positive ion is present in the lowest concentration?
(ii) Give the name of the compound formed from \(K^+\) and \(Cl^–\) ions.
(iii) Calculate the mass of potassium ions present in 200cm\(^3\) of this blood plasma.
mass of potassium ions = ………………………… g
(iv) When the \(1000~cm^3\) sample of blood plasma is crystallised, several compounds are formed.
Suggest the name of the compound which forms the greatest mass of crystals.
(b) Describe a test for potassium ions.
(c) Blood plasma also contains proteins. Proteins are present in food.
Which one of the following substances is also present in food?
Draw a circle around the correct answer.
carbohydrate hematite poly(ethene) terylene [1]
(d) Compound S is one of the monomer units used to make proteins. Its structure is shown.
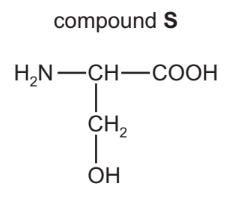
(i) On the structure, draw a circle around the alcohol functional group.
(ii) Deduce the molecular formula of compound S showing the number of carbon, hydrogen, oxygen and nitrogen atoms.
▶️ Answer/Explanation
(a)(i) Ans: \(Mg^{2+}\) (magnesium ion) has the lowest concentration (0.0015 mol/dm³).
(a)(ii) Ans: Potassium chloride (\(KCl\)) is formed from \(K^+\) and \(Cl^–\).
(a)(iii) Ans: Mass of \(K^+\) in 200 cm³ = \(\frac{0.16~g}{1000~cm^3} \times 200~cm^3 = 0.032~g\).
(a)(iv) Ans: Sodium chloride (\(NaCl\)) forms the greatest mass of crystals due to its highest concentration.
(b) Ans: Flame test for potassium ions produces a lilac flame.
(c) Ans: Carbohydrate is present in food (the other options are minerals or synthetic polymers).
(d)(i) Ans: The \(-OH\) group (alcohol functional group) should be circled.
(d)(ii) Ans: Molecular formula of compound S is \(C_3H_7NO_3\).
