(a) Describe how you could prepare a pure sample of crystals of hydrated copper(II) sulfate using dilute sulfuric acid and an excess of copper(II) oxide. [3]
(b) Anhydrous copper(II) sulfate is used to test for water.
\[ \begin{aligned} & \mathrm{CuSO}_4 + 5 \mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O} \\ & \text{anhydrous} \quad \text{hydrated} \\ & \text{copper(II) sulfate} \quad \text{copper(II) sulfate} \\ \end{aligned} \]
(i) What is meant by the symbol $\rightleftharpoons$? [1]
(ii) How can hydrated copper(II) sulfate be changed into anhydrous copper(II) sulfate? [1]
(c) Complete the table to calculate the relative formula mass of anhydrous copper(II) sulfate, $\mathrm{CuSO}_4$. Use your Periodic Table to help you.

relative formula mass = …………………………. [2]
(d) Complete the table to show the number of electrons, protons, and neutrons in the sulfur atom and copper ion shown.

(e) Alloys of copper are used to make coins.
(i) What is meant by the term alloy? [1]
(ii) Suggest why an alloy of copper is used to make coins instead of using pure copper. [1] [Total: 13]
▶️ Answer/Explanation
(a) Ans:
1. React excess copper(II) oxide with dilute sulfuric acid until no more dissolves.
2. Filter the mixture to remove unreacted copper(II) oxide.
3. Heat the filtrate to evaporate water until crystals form, then allow to cool and dry.
(b)(i) Ans: Reversible reaction
The symbol $\rightleftharpoons$ indicates that the reaction can proceed in both directions.
(b)(ii) Ans: Heating
Hydrated copper(II) sulfate loses water upon heating, converting to anhydrous form.
(c) Ans: 160
Calculated as: Cu (64) + S (32) + 4 × O (16 × 4 = 64) = 64 + 32 + 64 = 160.
(d) Ans:
Sulfur atom:
Protons = 16, Neutrons = 18, Electrons = 16.
Copper ion (Cu²⁺):
Protons = 29, Neutrons = 34, Electrons = 27 (since it loses 2 electrons).
(e)(i) Ans: A mixture of a metal with other elements
Alloys enhance properties like strength and durability.
(e)(ii) Ans: More resistant to wear and corrosion
Copper alloys are harder and more durable than pure copper, making them ideal for coins.
(a) The table shows the percentage by mass of the elements on Earth and in the Universe.
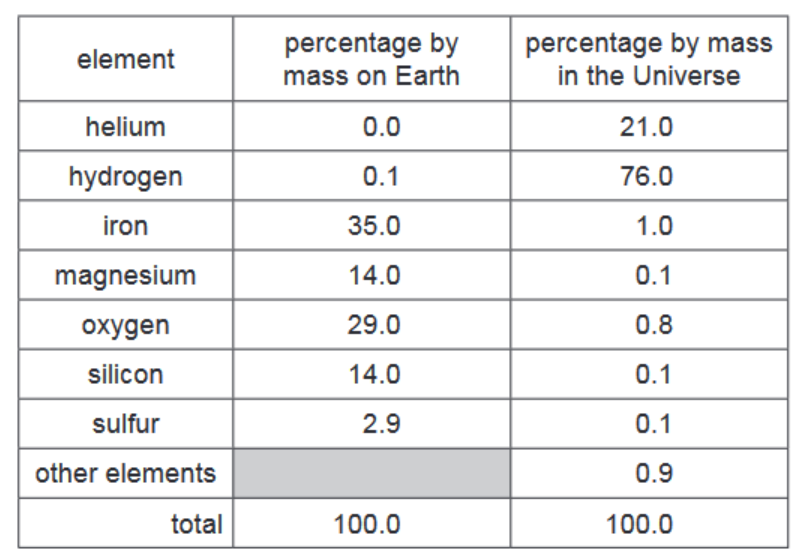
Answer these questions using only the information in the table.
(i) Deduce the percentage by mass of other elements present on Earth. [1]
(ii) Which non-metallic element is present on Earth in the greatest percentage by mass? [1]
(iii) Give two major differences in the percentage by mass of the elements on Earth and in the Universe. [2]
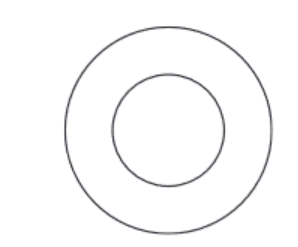
(b) Complete the diagram to show the electron arrangement in an oxygen atom.
(c) Helium, neon and argon are noble gases.
(i) Explain, in terms of the electronic structure, why neon is unreactive. [1]
(ii) State one use of argon. [1]
▶️ Answer/Explanation
(a)(i) 5.0% – Calculated by subtracting the given percentages (Fe 35%, O 30%, Si 15%, Mg 13%, S 2%) from 100%.
(a)(ii) Oxygen – With 30% composition, it’s the most abundant non-metal on Earth (metals like iron are more abundant but the question asks specifically for non-metals).
(a)(iii) Two key differences:
1. Hydrogen dominates the Universe (75%) but is rare on Earth (part of the 5% “others”)
2. Helium is second most abundant in Universe (23%) but negligible on Earth
(b) Electron arrangement: 2,6 – Two electrons in the first shell and six in the outer shell, totaling 8 electrons matching oxygen’s atomic number.
(c)(i) Neon has a complete outer electron shell (octet) with 8 electrons, making it energetically stable and unreactive as it neither gains nor loses electrons easily.
(c)(ii) Argon is used in light bulbs – Its inert nature prevents filament oxidation, extending bulb life. Alternatively, it’s used in welding to create inert atmospheres.
