The diagrams show part of the structures of five substances, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ and $\mathbf{E}$.

(a) Answer the following questions about these structures.
Each structure may be used once, more than once or not at all.

(i) Which two of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, are covalently bonded? [2]
(ii) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is a diatomic molecule? [1]
(iii) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is a compound? [1]
(iv) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is very soluble in water? [1]
(v) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is used in cutting tools? [1]
(vi) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is used in electrical wiring? [1]
(b) Substance $\mathbf{B}$ is an element.
What is meant by the term element? [1]
▶️ Answer/Explanation
(a)(i) Ans: B and D
B (diamond) has a giant covalent structure, and D (N₂) has a simple covalent bond between two nitrogen atoms.
(a)(ii) Ans: D
D (N₂) is a diatomic molecule consisting of two nitrogen atoms bonded together.
(a)(iii) Ans: C
C (LiCl) is a compound because it contains lithium and chlorine chemically bonded together.
(a)(iv) Ans: C
C (LiCl) is very soluble in water due to its ionic nature, which allows it to dissociate into ions.
(a)(v) Ans: B
B (diamond) is used in cutting tools because of its extreme hardness.
(a)(vi) Ans: E
E (copper) is used in electrical wiring because it is an excellent conductor of electricity.
(b) Ans: An element is a substance in which all the atoms have the same proton number or a substance containing only one type of atom.
Some properties of four substances, A, B, C and D, are shown in the table.
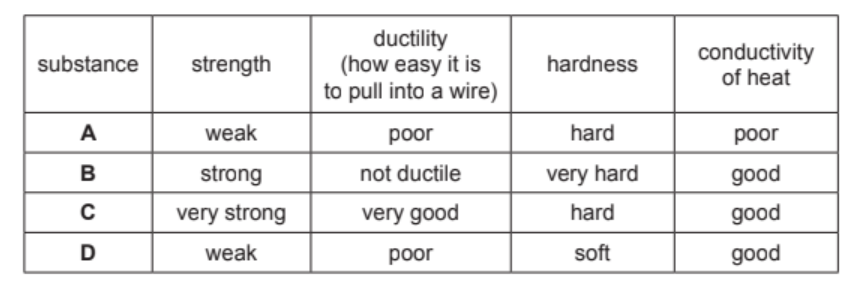
Answer these questions using only the information in the table.
(a) State which substance, A, B, C or D, is best used in the core of an overhead electricity cable.
Explain your answer.
(b) State which substance, A, B, C or D, is best used for the tip of a drill.
Explain your answer.
▶️ Answer/Explanation
(a) Ans: C
For overhead cables, we need a material with high strength to withstand tension and good ductility for flexibility. Substance C has “very strong” strength (9/10) and the best ductility (9/10) among all options, making it ideal for cable cores where both properties are crucial.
(b) Ans: B
Drill tips require extreme hardness to cut through materials and good heat conductivity to dissipate heat. Substance B scores highest in hardness (9/10) and has good heat conductivity (7/10), while maintaining adequate strength (7/10) – perfect for withstanding drilling forces.
