The structure of an organic compound is shown.
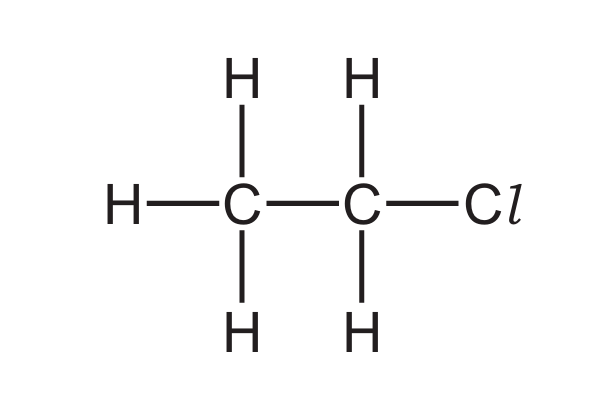
What is the name of the compound?
A) chloroethane
B) chloroethene
C) chloroethanol
D) chloroethanoic acid
▶️ Answer/Explanation
Ans: A
The structure shows a two-carbon chain with single bonds (ethane) with one chlorine substituent:
CH3-CH2Cl
This is chloroethane. Key points:
– It’s not an alkene (no double bond) so not chloroethene
– No OH group present (rules out chloroethanol)
– No carboxyl group (rules out chloroethanoic acid)
The correct IUPAC name is chloroethane.
Question
Which structure is correctly named?

Answer/Explanation
Ans:
C
Question
Some properties of an organic compound J are listed.
● It is a liquid at room temperature.
● It is soluble in water.
● A solution of J reacts with calcium carbonate to form carbon dioxide.
● A solution of J has a pH of 3.
In which homologous series does J belong?
A alkane
B alkene
C alcohol
D carboxylic acid
▶️Answer/Explanation
Ans:D
Question
When the alcohol \(CH_3CH_2CH_2OH\) reacts with the carboxylic acid \(CH_3CH_2CH_2COOH\) an ester is formed.
What is the name and structural formula of this ester?
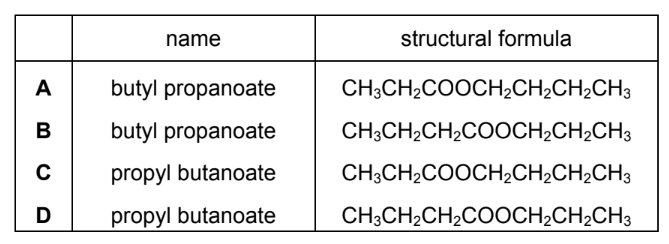
Answer/Explanation
Ans:D
