Ethene reacts with steam to produce ethanol.
Which row describes each compound?
| ethene | ethanol | |
|---|---|---|
| A | saturated | saturated |
| B | saturated | unsaturated |
| C | unsaturated | saturated |
| D | unsaturated | unsaturated |
▶️ Answer/Explanation
Ans: C
Ethene (C2H4) is unsaturated because it contains a C=C double bond. When it reacts with steam (H2O), the product ethanol (C2H5OH) is saturated because all carbon-carbon bonds are single bonds. Therefore, the correct description is ethene = unsaturated, ethanol = saturated, which is option C.
Information about two reactions of ethene is listed.
- Reaction 1 requires a nickel catalyst.
- Reaction 2 requires an acid catalyst.
Which substance reacts with ethene in each reaction?
| reaction 1 | reaction 2 | |
|---|---|---|
| A) | bromine | steam |
| B) | bromine | hydrogen |
| C) | hydrogen | bromine |
| D) | hydrogen | steam |
▶️ Answer/Explanation
Ans: D
Let’s analyze the reactions:
Reaction 1 (nickel catalyst): This is hydrogenation, where hydrogen adds across the double bond of ethene to form ethane. Nickel is a common hydrogenation catalyst.
Reaction 2 (acid catalyst): This is hydration, where steam (water) adds to ethene to form ethanol. Phosphoric acid is typically used as the catalyst.
Therefore, the correct combination is:
– Reaction 1: hydrogen (with nickel catalyst)
– Reaction 2: steam (with acid catalyst)
This matches option D. The other options are incorrect because:
– Bromine reactions with ethene don’t require these catalysts (they occur readily at room temperature)
– Hydrogen doesn’t react with ethene without a nickel catalyst
Bromine reacts with but-2-ene.
What is the displayed formula of the product of this reaction?
A) 
B) 
C) 
D) 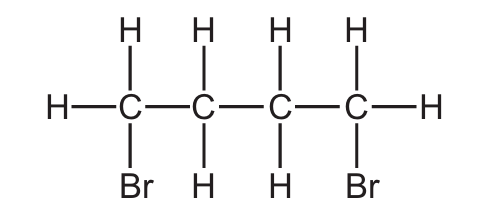
▶️ Answer/Explanation
Ans: C
This is an electrophilic addition reaction of bromine with but-2-ene (CH3CH=CHCH3). The reaction proceeds as follows:
- The π electrons in the C=C double bond attack a bromine molecule, forming a bromonium ion intermediate
- The second bromine ion then attacks from the opposite side (anti-addition)
- The product is 2,3-dibromobutane
The correct structure shows two bromine atoms added to the second and third carbon atoms (the original double bond positions) in an anti configuration. The methyl groups (CH3) remain on the first and fourth carbons. Option C correctly shows this arrangement with the bromine atoms on adjacent carbons in a trans configuration (dashed and wedged bonds indicating they’re on opposite sides).
Which equation represents an addition reaction?
A) CH3CHO + HCN → CH3CH(OH)CN
B) C6H6 + Br2 → C6H5Br + HBr
C) NH4Br → NH3 + HBr
D) C14H30 → C2H4 + C8H18 + C4H8
▶️ Answer/Explanation
Ans: A
An addition reaction is where two or more molecules combine to form a single product with no other products.
1. Option A: This shows the addition of HCN to ethanal (CH3CHO) forming a single product (correct answer).
2. Option B: This is a substitution reaction where Br replaces H in benzene.
3. Option C: This is a decomposition reaction where NH4Br breaks down.
4. Option D: This is cracking, a type of decomposition reaction.
Only option A shows two reactants combining to form one product with all atoms incorporated into the final molecule.
