The table shows two methods used to make ethanol.
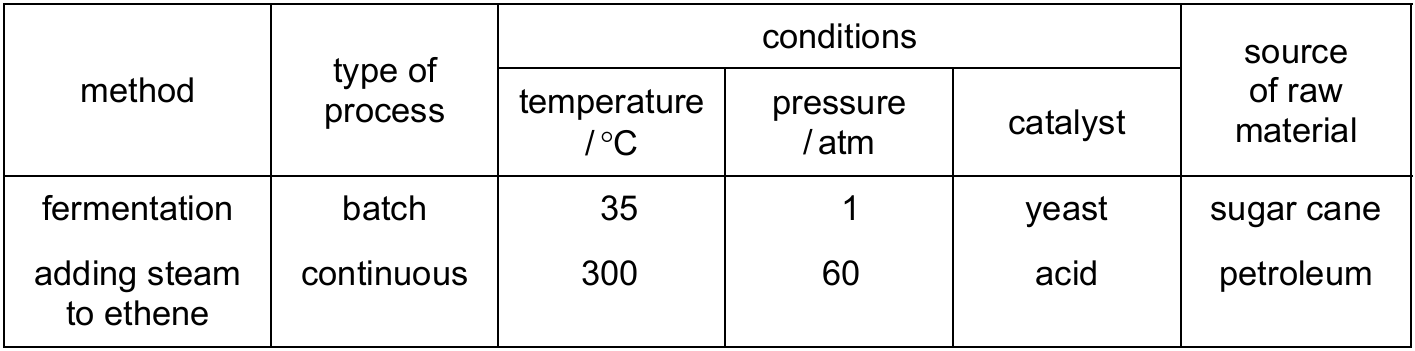
Which statement gives an advantage of preparing ethanol by fermentation rather than by adding steam to ethene?
A) Fermentation takes several days to complete.
B) Little energy is used in the fermentation process.
C) The fermentation of glucose from sugar cane produces pure ethanol.
D) Fermentation uses a non-renewable raw material.
▶️ Answer/Explanation
Ans: B
Comparing the two methods:
1. Fermentation advantages:
– Uses renewable resources (sugar cane) (option D is incorrect)
– Operates at low temperature and pressure (option B is correct)
– Doesn’t require high energy inputs
2. Hydration of ethene disadvantages:
– Requires high temperature and pressure (energy intensive)
– Uses non-renewable petroleum
Option A describes a disadvantage of fermentation. Option C is incorrect as fermentation produces a mixture that needs distillation.
Question
Which statements about ethanol are correct?
1 Ethanol is used as a solvent.
2 Ethanol can be made directly from ethane.
3 Ethanol is a covalent compound.
A. 1 only B. 1 and 2 C. 1 and 3 D. 2 and 3
Answer/Explanation
Ans:
C
Question
The reaction of ethanol with acidified potassium manganate(VII) is shown.
\(CH_{3}CH_{2}OH\xrightarrow[H^{+}]{KMnO_{4}}CH_{3}COOH\)
Which type of reaction is taking place?
- addition
- condensation
- hydrolysis
- oxidation
Answer/Explanation
Ans:
D
Question
Ethanol is made industrially by the fermentation of glucose or by the catalytic addition of steam to ethene.
Which statement describes an advantage of fermentation compared to catalytic addition?
- Ethanol is the only product of fermentation.
- Fermentation uses a batch process but catalytic addition is continuous.
- Fermentation uses a higher temperature than catalytic addition.
- Fermentation uses a renewable resource.
Answer/Explanation
Ans:
D
