Which diagram represents one helium atom?
A) 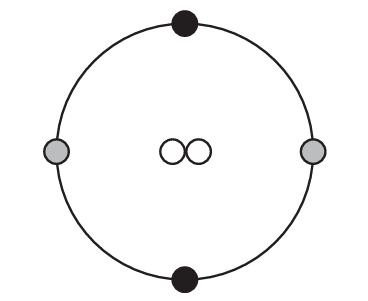
B) 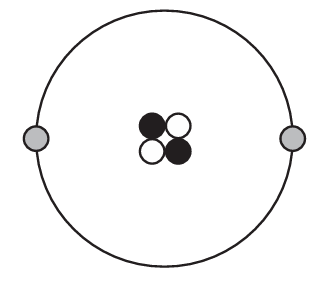
C) 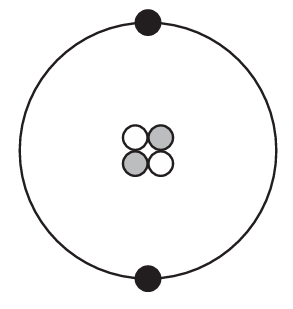
D) 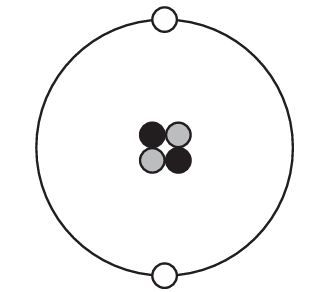
▶️ Answer/Explanation
Ans: B
A helium atom has an atomic number of 2, meaning it has 2 protons in its nucleus. The most common isotope (helium-4) has 2 neutrons as well, giving it a mass number of 4. It has 2 electrons in its first and only electron shell.
The correct diagram (B) would show:
– A nucleus containing 2 protons and 2 neutrons
– 2 electrons in the first electron shell
Other options likely show incorrect configurations (wrong number of particles or incorrect electron arrangement).
Question
The numbers of protons and neutrons and the electronic structures of four particles, W, X, Y and Z, are shown.

Which particles have the same chemical properties?
A W and Y B W and Z C X and Y D X and Z
Answer/Explanation
Ans:
C
Question
Lithium and fluorine react to form lithium fluoride.
A student writes three statements about the reaction.
1 Lithium atoms lose an electron when they react.
2 Each fluoride ion has one more electron than a fluorine atom.
3 Lithium fluoride is a mixture of elements.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: A
Question
Element X has 7 protons.
Element Y has 8 more protons than X.
Which statement about element Y is correct?
A Y has more electron shells than X.
B Y has more electrons in its outer shell than X.
C Y is in a different group of the Periodic Table from X.
D Y is in the same period of the Periodic Table as X.
Answer/Explanation
Ans: A
