Calcium carbonate is heated. Calcium oxide and carbon dioxide gas are formed.
The equation for the reaction is shown.
\[ \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \]
225 kg of calcium carbonate is heated until there is no further change in mass.
The yield of calcium oxide is 85 kg.
What is the percentage yield?
A) 37.8%
B) 47.2%
C) 67.5%
D) 85.0%
▶️ Answer/Explanation
Ans: C
First calculate the theoretical yield:
1. Molar mass of CaCO3 = 40 + 12 + (16×3) = 100 g/mol
2. Moles of CaCO3 = 225,000 g / 100 g/mol = 2,250 mol
3. From the equation, 1 mol CaCO3 produces 1 mol CaO
4. Molar mass of CaO = 40 + 16 = 56 g/mol
5. Theoretical yield = 2,250 mol × 56 g/mol = 126,000 g = 126 kg
Now calculate percentage yield:
Percentage yield = (actual yield / theoretical yield) × 100
= (85 kg / 126 kg) × 100 ≈ 67.5%
The equation for the decomposition of ammonium carbonate, \((\text{NH}_4)_2\text{CO}_3\), is shown.
\[(\text{NH}_4)_2\text{CO}_3(s) \rightarrow 2\text{NH}_3(g) + \text{CO}_2(g) + \text{H}_2\text{O}(l)\]
[\(M: (\text{NH}_4)_2\text{CO}_3, 96\)]
The total volume of gas produced is 360 cm\(^3\) at r.t.p.
Which mass of ammonium carbonate, \((\text{NH}_4)_2\text{CO}_3\), is decomposed?
A) 0.24 g
B) 0.48 g
C) 0.96 g
D) 1.44 g
▶️ Answer/Explanation
Ans: B
1. First calculate total moles of gas produced: 360 cm³ = 0.36 dm³. At r.t.p., 1 mole occupies 24 dm³, so moles of gas = 0.36/24 = 0.015 mol.
2. From the equation: 1 mole \((\text{NH}_4)_2\text{CO}_3\) produces 3 moles of gas (2 NH₃ + 1 CO₂).
3. So moles of \((\text{NH}_4)_2\text{CO}_3\) decomposed = 0.015/3 = 0.005 mol.
4. Mass = moles × Mr = 0.005 × 96 = 0.48 g.
Note: H₂O is liquid and doesn’t contribute to gas volume at r.t.p.
The structure of ethene is shown.

How many hydrogen atoms and how many carbon atoms are in one mole of ethene?
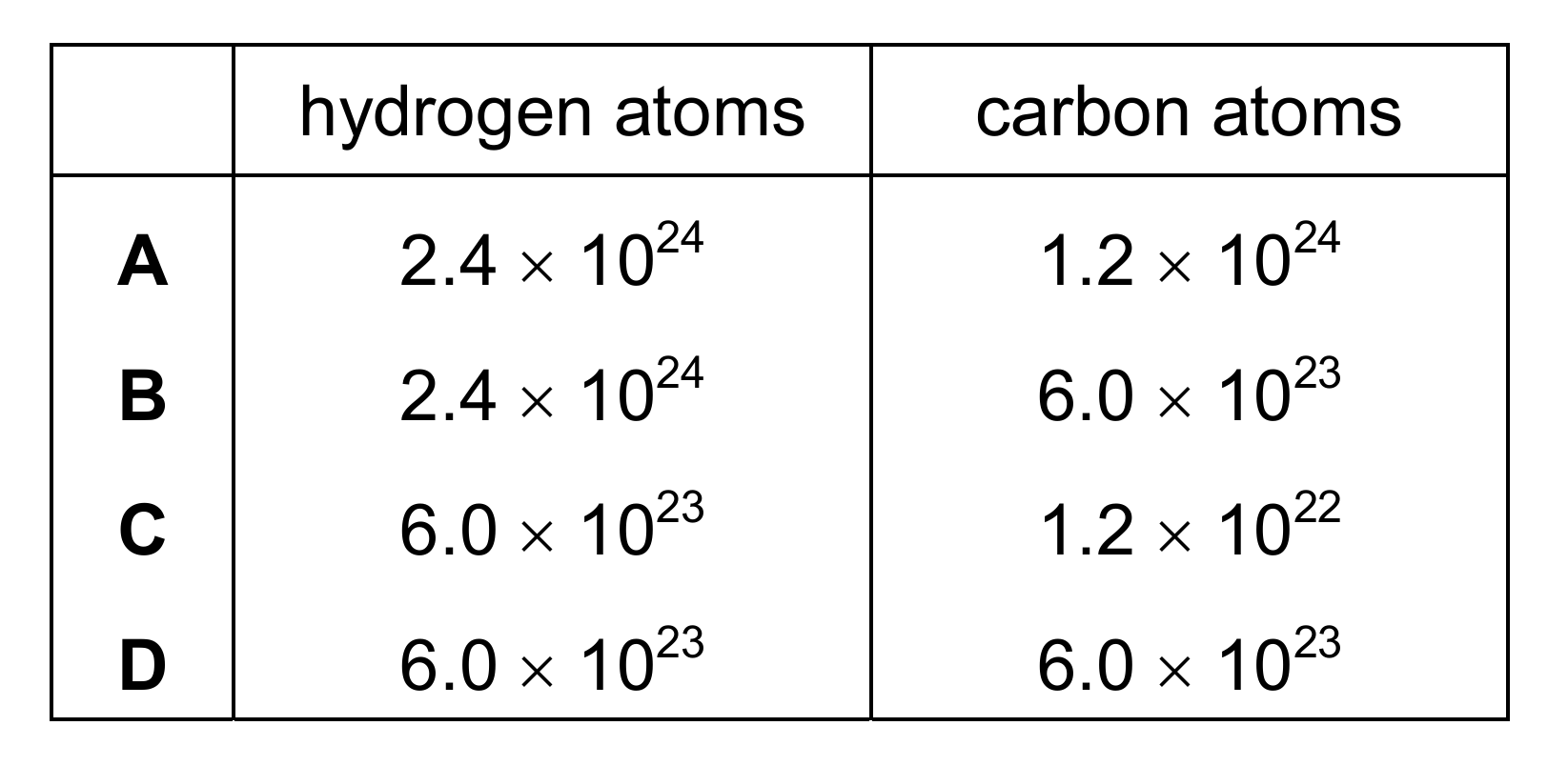
▶️ Answer/Explanation
Ans: A
One mole of ethene (C2H4) contains 2 moles of carbon atoms and 4 moles of hydrogen atoms. Using Avogadro’s number (6.0 × 1023 particles per mole):
Carbon atoms: 2 × 6.0 × 1023 = 1.2 × 1024
Hydrogen atoms: 4 × 6.0 × 1023 = 2.4 × 1024
Therefore, option A is correct. The other options either miscalculate the number of atoms or don’t account for the molecular formula properly.
Question
The equation for the reaction between aqueous lead(II) nitrate and aqueous sodium chloride is shown.
Pb(NO3)2(aq) + 2NaCl(aq) → PbCl2(s) + 2NaNO3(aq)
If 100 cm3 of aqueous lead(II) nitrate of concentration 0.1 mol / dm3 is reacted with an excess of aqueous sodium chloride, which mass of lead(II) chloride is obtained?
A 1.16 g B 2.42 g C 2.78 g D 3.31 g
Answer/Explanation
Ans:
C
