The equation for the reaction of carbon with carbon dioxide is shown.
\[ C + CO_2 \rightarrow 2CO \]
Which row identifies the carbon atom that is reduced and its change in oxidation number?
| atom that is reduced | change in oxidation number | |
|---|---|---|
| A | carbon in CO2 | +2 → +4 |
| B | carbon in CO2 | +4 → +2 |
| C | elemental carbon, C | 0 → +2 |
| D | elemental carbon, C | +2 → 0 |
▶️ Answer/Explanation
Ans: B
1. In CO2, carbon has oxidation number +4 (oxygen is -2 each).
2. In CO, carbon has oxidation number +2 (oxygen is -2).
3. Elemental carbon (C) has oxidation number 0.
4. The carbon in CO2 is reduced (oxidation number decreases from +4 to +2).
5. Elemental carbon is oxidized (oxidation number increases from 0 to +2).
Therefore, the correct answer is B, showing the reduction of carbon in CO2 with the correct change in oxidation number.
Aqueous iron(II) sulfate is added to acidified potassium manganate(VII). The purple colour of the potassium manganate(VII) disappears.
Aqueous potassium iodide is added to acidified potassium dichromate(VI). A dark brown solution forms.
Which row identifies the role of the iron(II) sulfate and the potassium dichromate(VI) in these reactions?
| iron(II) sulfate | potassium dichromate(VI) | |
|---|---|---|
| A | oxidising agent | oxidising agent |
| B | oxidising agent | reducing agent |
| C | reducing agent | reducing agent |
| D | reducing agent | oxidising agent |
▶️ Answer/Explanation
Ans: D
1. In the first reaction, iron(II) sulfate causes the purple manganate(VII) to decolorize, meaning Fe2+ is oxidized to Fe3+, so it acts as a reducing agent.
2. In the second reaction, potassium dichromate(VI) oxidizes iodide ions (I–) to iodine (I2, which causes the brown color), so it acts as an oxidizing agent.
3. Therefore, iron(II) sulfate is the reducing agent and potassium dichromate(VI) is the oxidizing agent.
Which statement about reactants in redox reactions is correct?
A) An oxidising agent donates electrons, and a reducing agent accepts electrons.
B) When one element gains electrons, the oxidation number of a different element increases.
C) When the oxidation number of one element increases, a different element gains oxygen.
D) When the oxidation number of one element increases, a different element loses electrons.
▶️ Answer/Explanation
Ans: B
In redox reactions, when one species gains electrons (is reduced), another must lose electrons (be oxidized). This means the oxidation number of the element that loses electrons increases (B is correct). Option A is wrong because oxidising agents accept electrons, not donate. Options C and D are incorrect generalizations – oxygen gain or electron loss aren’t necessary for all redox reactions, but oxidation number changes are fundamental.
Aluminium is extracted from aluminium oxide by electrolysis. The ionic half-equation for the reaction at one of the electrodes is shown.
\[ \text{Al}^{3+} + 3\text{e}^- \rightarrow \text{Al} \]
Which row describes the change in oxidation number of the aluminium and the type of reaction at this electrode?
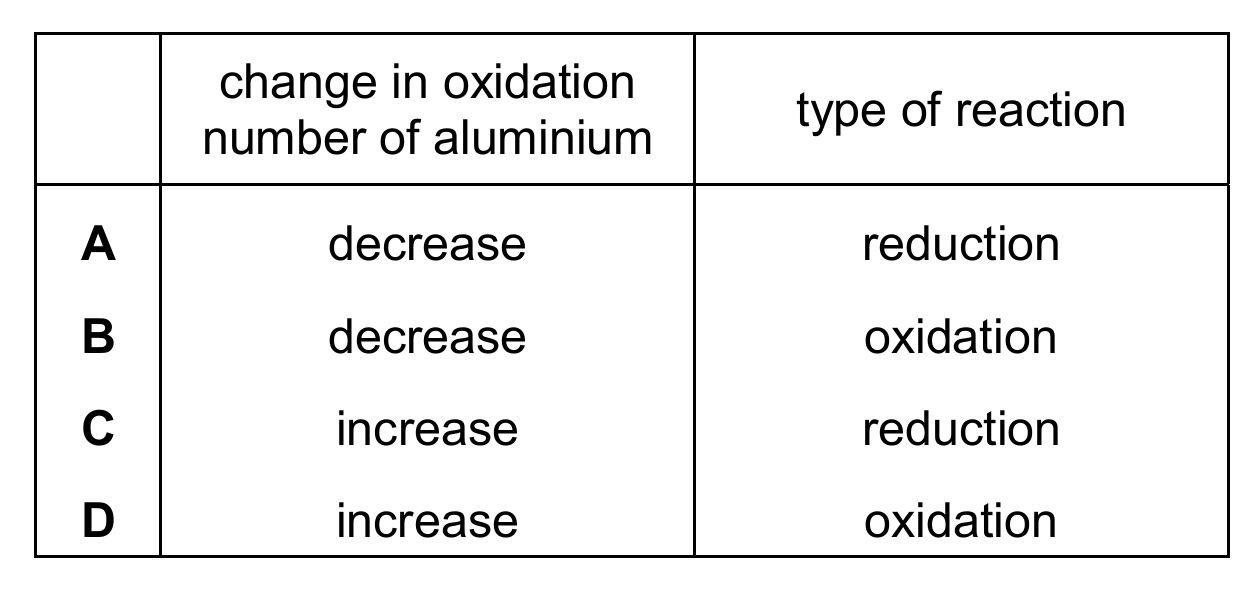
▶️ Answer/Explanation
Ans: A
The half-equation shows Al³⁺ gaining 3 electrons to become Al. This is reduction (gain of electrons), and the oxidation number decreases from +3 to 0. Therefore, option A is correct. Reduction always involves a decrease in oxidation number. Options B and D incorrectly identify the reaction type, while option C incorrectly states the oxidation number increases during reduction.
