Copper(II) sulfate is made when copper(II) carbonate reacts with dilute sulfuric acid.
\[ \text{CuCO}_3 + \text{H}_2\text{SO}_4 \rightarrow \text{CuSO}_4 + \text{H}_2\text{O} + \text{CO}_2 \]
Pure copper(II) sulfate crystals are obtained.
Which reagent is in excess and how are the crystals obtained?
| reagent in excess | how the crystals are obtained | |
|---|---|---|
| A | copper(II) carbonate | filter and evaporate the solution to dryness |
| B | copper(II) carbonate | filter, evaporate the solution to crystallising point and then cool |
| C | dilute sulfuric acid | evaporate the solution to dryness |
| D | dilute sulfuric acid | evaporate the solution to crystallising point and then cool |
▶️ Answer/Explanation
Ans: B
Copper(II) carbonate is insoluble, so it’s typically added in excess to ensure all the acid reacts. The excess solid can then be removed by filtration. To obtain pure crystals, the solution should be evaporated to the crystallising point (not to dryness, which would produce an impure powder) and then cooled to allow crystals to form. This method gives larger, purer crystals than evaporating to dryness.
Which row describes and gives the formula of hydrated copper(II) sulfate?
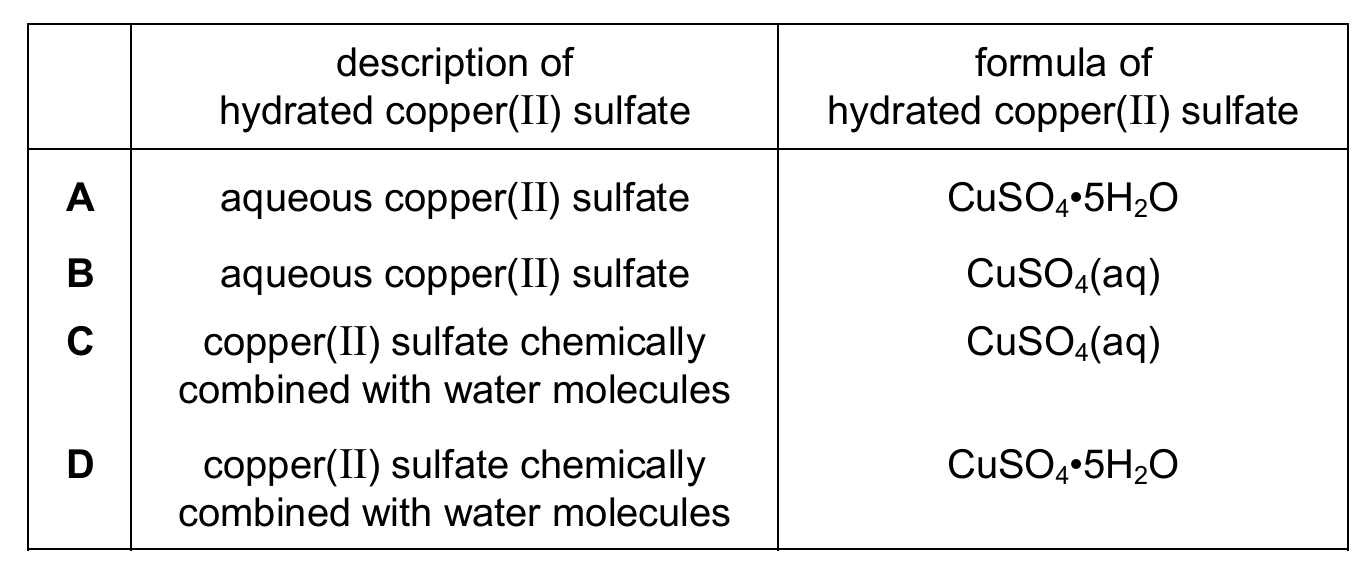
▶️ Answer/Explanation
Ans: D
Hydrated copper(II) sulfate refers to the solid compound where water molecules are chemically bound in the crystal structure (not just dissolved in water). Its correct formula is CuSO₄•5H₂O (the dot indicates water of crystallization). Therefore, option D is correct. Options A and B incorrectly describe it as aqueous (dissolved in water), while option C uses the wrong formula for the hydrated solid.
The equations for three reactions are shown.
1 Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq)
2 2AgNO₃(aq) + CuI₂(aq) → Cu(NO₃)₂(aq) + 2AgI(s)
3 CuO(s) + H₂SO₄(aq) → CuSO₄(aq) + H₂O(l)
Which reactions are suitable for making a salt by precipitation?
A) 1 and 2 only
B) 1 and 3 only
C) 2 and 3 only
D) 1, 2 and 3
▶️ Answer/Explanation
Ans: A
Precipitation reactions involve the formation of an insoluble solid (precipitate) when two solutions are mixed. In reaction 1, PbI₂ is the precipitate. In reaction 2, AgI is the precipitate. Reaction 3 is an acid-base neutralization producing a soluble salt (CuSO₄) and water – no precipitate forms. Therefore, only reactions 1 and 2 are precipitation reactions (option A).
Question
Information about some silver compounds is shown.

Which equation shows a reaction which cannot be used to make a silver salt?
A. \(AgNO_{3}(aq)+HC\imath (aq)\rightarrow AgC\imath (s)+HNO_{3}(aq)\)
B. \(Ag_{2}O(s)+2HNO_{3}(aq)\rightarrow 2AgNO_{3}(aq)+H_{2}O(l)\)
C. \(Ag_{2}CO_{3}(s)+2HNO_{3}(aq)\rightarrow 2AgNO_{3}(aq)+H_{2}O(l)+CO_{2}(g)\)
D. \(2Ag(s)+2HC\imath (aq)\rightarrow 2AgC\imath (s)+H_{2}(g)\)
Answer/Explanation
Ans:
D
