Which statements describe the Periodic Table?
1 The elements are arranged in order of their nucleon number.
2 The elements are arranged in order of their proton number.
3 It is used to predict the properties of elements.
A) 1 and 3
B) 1 only
C) 2 and 3
D) 2 only
▶️ Answer/Explanation
Ans: C
The Periodic Table arranges elements by increasing atomic (proton) number (statement 2 is correct, 1 is wrong). The periodic arrangement allows prediction of element properties based on their position (statement 3 is correct). Therefore, option C (2 and 3) is correct. Nucleon number (mass number) isn’t the basis for the periodic arrangement, as isotopes would disrupt the periodic trends if it were.
Question
Elements in Group IV of the Periodic Table are shown.
carbon
silicon
germanium
tin
lead
What does not occur in Group IV as it is descended?
- The proton number of the elements increases.
- The elements become more metallic.
- The elements have more electrons in their outer shell.
- The elements have more electron shells.
Answer/Explanation
Ans:
C
Question
The table gives some properties of Group IV elements.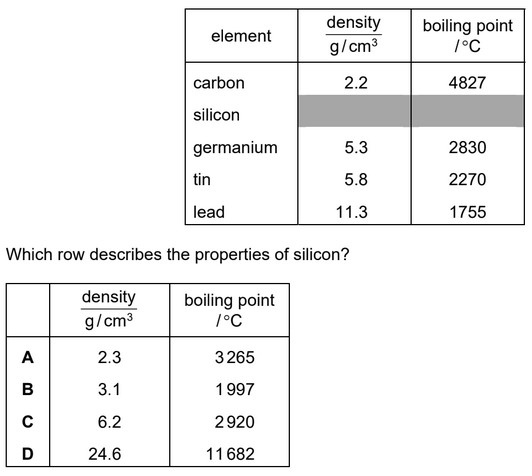
Answer/Explanation
Ans: A
Question
Which row describes the reactions of magnesium hydroxide and magnesium oxide?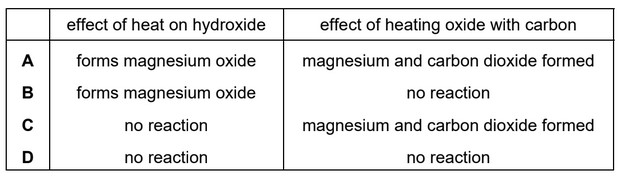
Answer/Explanation
Ans: B
