Which statement about elements in Group I or Group VII of the Periodic Table is correct?
A) Bromine reacts with potassium chloride to produce chlorine.
B) Iodine is a monatomic non-metal.
C) Lithium has a higher melting point than potassium.
D) Sodium is more reactive with water than potassium.
▶️ Answer/Explanation
Ans: C
In Group I (alkali metals), melting points decrease down the group, so lithium (higher in the group) has a higher melting point than potassium. Option A is incorrect because bromine is less reactive than chlorine and can’t displace it. Option B is wrong as iodine exists as diatomic I₂ molecules. Option D is incorrect because reactivity increases down Group I, making potassium more reactive than sodium.
Question
Metal X reacts with non-metal Y to form an ionic compound with the formula \(X_2Y\).
Which statements are correct?
1 X is in Group I of the Periodic Table.
2 X is in Group II of the Periodic Table.
3 Y is in Group VI of the Periodic Table.
4 Y is in Group VII of the Periodic Table.
A 1 and 3 B 1 and 4 C 2 and 3 D 2 and 4
Answer/Explanation
Ans: A
Question
Some properties of two metals, G and H, are shown.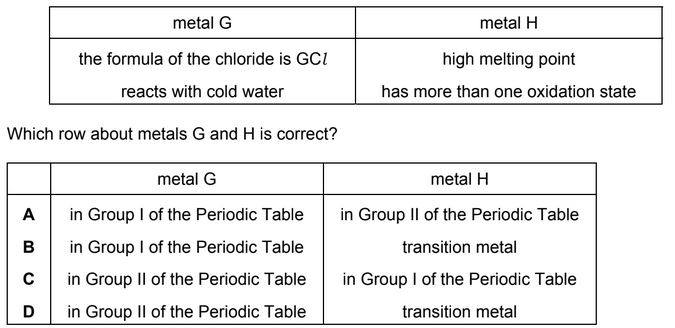
Answer/Explanation
Ans: B
Question
The properties of an element are shown.
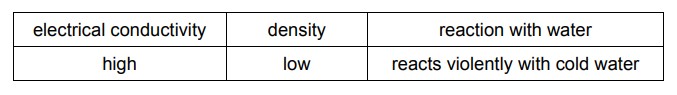
Which element has these properties?
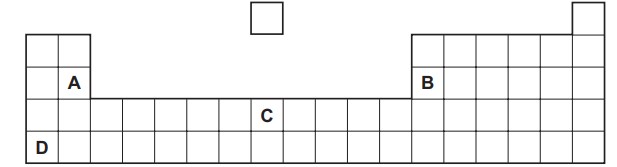
Answer/Explanation
Ans:
D
