Manganese(IV) oxide, MnO2, is a black solid.
The equation for the reaction between manganese(IV) oxide and dilute hydrochloric acid is shown.
\[ \text{MnO}_2 + 4\text{HCl} \rightarrow \text{MnCl}_2 + 2\text{H}_2\text{O} + \text{Cl}_2 \]
The reaction produces a pale pink solution.
Which properties of transition elements does this reaction show?
- They can act as catalysts.
- They form coloured compounds.
- They have high melting points.
- They have variable oxidation numbers.
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: D
Let’s analyze each property:
1. Catalytic ability – Not demonstrated in this reaction (MnO2 is a reactant, not a catalyst)
2. Colored compounds – The pale pink solution (Mn2+ ions) shows this property
3. High melting points – While true, this isn’t shown in the reaction
4. Variable oxidation numbers – Mn changes from +4 (MnO2) to +2 (MnCl2)
Therefore, only properties 2 and 4 are demonstrated in this reaction.
Which row shows the properties of a transition element?
| catalyst | colour of oxide | electrical conductivity | |
|---|---|---|---|
| A | yes | red | good |
| B | yes | green | poor |
| C | no | yellow | good |
| D | no | white | poor |
▶️ Answer/Explanation
Ans: A
Transition elements typically act as catalysts (e.g., iron in the Haber process), form colored compounds (red oxide in this case), and are good conductors of electricity due to their metallic bonding and delocalized electrons. These are all characteristic properties of transition metals.
Acidified potassium dichromate(VI), K₂Cr₂O₇, is used to oxidise ethanol, C₂H₅OH.
The ionic equation for the reaction is shown.
\[ 3C_2H_5OH + 2Cr_2O_7^{2-} + 16H^+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O \]
Which properties of transition elements are shown by chromium in this reaction?
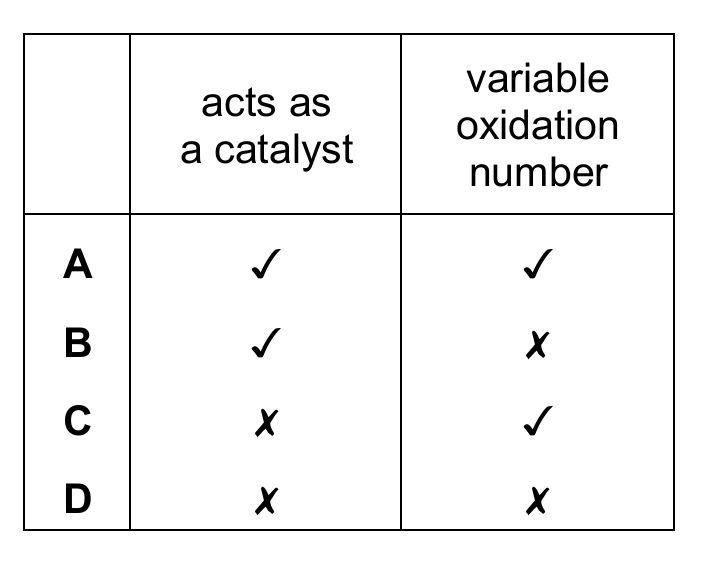
▶️ Answer/Explanation
Ans: C
In this reaction, chromium changes from Cr₂O₇²⁻ (Cr oxidation number +6) to Cr³⁺, showing variable oxidation number (✓). However, potassium dichromate is not acting as a catalyst here – it’s being used up in the reaction (✗). Therefore, option C is correct. Transition elements often show variable oxidation states, but not all their compounds act as catalysts in every reaction they participate in.
Question
The table gives some properties of an element.

Which other property does this element have?
A. acts as a catalyst
B. brittle
C. forms an acidic oxide
D. highly reactive with water
Answer/Explanation
Ans:
A
