The apparatus used for the extraction of aluminium by electrolysis is shown.
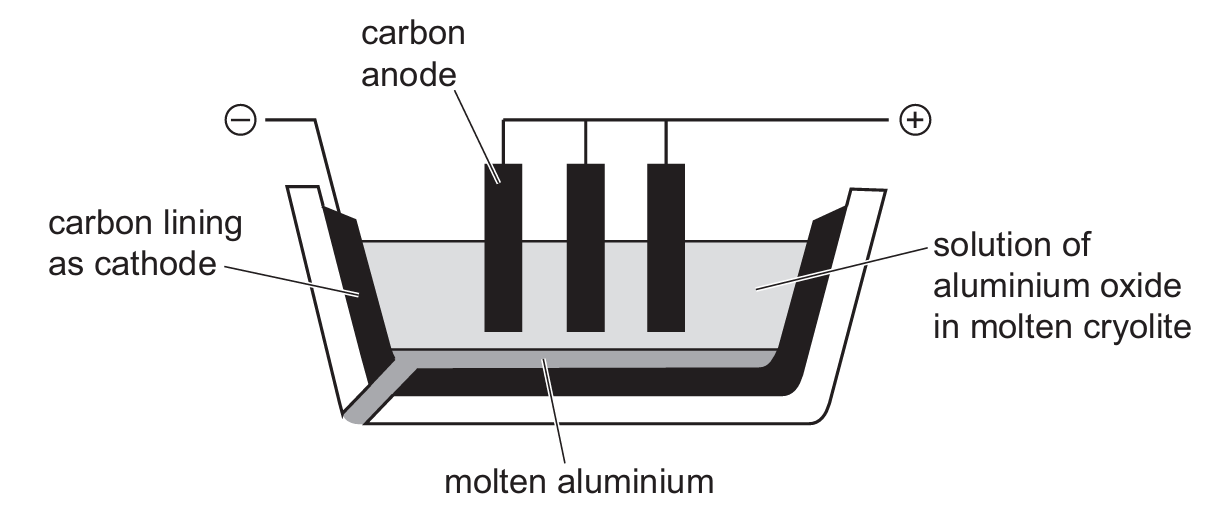
Which equation represents the reaction at the anode?
A) \( O + 2e^- \rightarrow O^{2-} \)
B) \( 2O^{2-} \rightarrow O_2 + 4e^- \)
C) \( Al^{3-} \rightarrow Al + 3e^- \)
D) \( Al^{3+} + 3e^- \rightarrow Al \)
▶️ Answer/Explanation
Ans: B
In aluminium extraction:
At the cathode: Al3+ + 3e– → Al (reduction)
At the anode: Oxygen ions are oxidized:
2O2- → O2 + 4e– (option B)
Other options are incorrect because:
A: Shows reduction (gaining electrons) at anode – wrong
C: Incorrect ion (Al3- doesn’t exist) and shows oxidation at cathode – wrong
D: Shows cathode reaction, not anode
The carbon anode reacts with the oxygen produced, forming CO2, but the initial oxidation is of oxide ions.
Aluminium is extracted from its ore by electrolysis.
What is the role of cryolite in this process?
A) to lower the operating temperature
B) to lower the boiling point of bauxite
C) to raise the melting point of bauxite
D) to act as a catalyst
▶️ Answer/Explanation
Ans: A
Cryolite (Na3AlF6) is added to the alumina (Al2O3) in the Hall-Héroult process for aluminum extraction. The main purpose of cryolite is to lower the melting point of the alumina from about 2072°C to around 950°C, which significantly reduces the energy required for the electrolysis process. This makes the extraction more economically viable. Cryolite doesn’t act as a catalyst (it’s not regenerated), nor does it affect the boiling point of bauxite. It specifically lowers the operating temperature of the electrolytic cell.
Question
Which statement about the extraction of aluminium is correct?
A. Aluminium is formed at the cathode during the electrolysis of aluminium oxide.
B. Hematite is mainly aluminium oxide.
C. Molten cryolite is used to raise the melting point of the aluminium oxide.
D. Oxygen gains electrons at the anode during the electrolysis of aluminium oxide.
Answer/Explanation
Ans:
A
Question
In the extraction of aluminum by electrolysis, cryolite is added to the bauxite ore.
Which row describes the role of cryolite and gives the ionic half-equation at the cathode?

Answer/Explanation
Ans:
C
