Many organic compounds contain carbon, hydrogen and oxygen only.
(a) An organic compound V has the following composition by mass.
C, 48.65%; H, 8.11%; O, 43.24%
Calculate the empirical formula of compound V.
empirical formula =
(b) Compound W has the empirical formula CH4O and a relative molecular mass of 32.
Calculate the molecular formula of compound W.
molecular formula =
(c) Compounds X and Y have the same general formula.
X and Y are both carboxylic acids.
Compound X has the molecular formula C2H4O2.
Compound Y has the molecular formula C4H8O2.
(i) Deduce the general formula of compounds X and Y.
(ii) Draw the structure of compound Y. Show all of the atoms and all of the bonds.
Name compound Y.
(iii) Give the name used to describe a ‘family’ of similar compounds with the same general
formula, similar chemical properties and the same functional group.
(d) Propene is an unsaturated hydrocarbon. The formula of propene is shown.
CH3CH=CH2
(i) State the colour change observed when propene is added to aqueous bromine.
(ii) Propene can be produced by cracking long chain alkanes.
Pentadecane, C15H32, is cracked to produce an alkane and propene in a 1:2 molar ratio.
Complete the chemical equation for this reaction.
C15H32 → ………………………………… + …………………………………
(iii) Propene can be converted into poly(propene).
Name the type of polymerisation that occurs when propene is converted into poly(propene).
(iv) Complete the diagram to show a section of poly(propene).
▶️ Answer/Explanation
(a) Empirical formula calculation:
– Convert % to moles: C (48.65/12 = 4.05), H (8.11/1 = 8.11), O (43.24/16 = 2.70)
– Divide by smallest (2.70): C1.5H3O1
– Multiply by 2 to get whole numbers: C3H6O2
(b) Molecular formula:
– Empirical formula mass (CH4O) = 12 + 4 + 16 = 32
– Since Mr = 32, molecular formula = CH4O (methanol)
(c) (i) General formula: CnH2nO2 (for carboxylic acids)
(ii) Structure of Y (butanoic acid):
Name: butanoic acid
(iii) Family: homologous series
(d) (i) Bromine test: brown → colourless (due to addition across C=C bond)
(ii) Cracking equation: C15H32 → C9H20 (nonane) + 2C3H6 (propene)
(iii) Polymerisation type: addition polymerisation
(iv) Poly(propene) structure:
(a) Ethane, propane and butane are members of the same homologous series.
(i) Name this homologous series.
(ii) State two ways members of the same homologous series are similar.
(b) One mole of ethane, \(\mathrm{C}_2 \mathrm{H}_6\), contains \(6.02 \times 10^{23}\) molecules.
Calculate how many molecules are in \(15 \mathrm{~g}\) of ethane.
number of ethane molecules =
(c) Propane reacts with chlorine.
(i) Write the formula of the product which does not contain carbon.
(ii) Draw the structure of an organic product formed. Show all of the atoms and all of the bonds.
(iii) State the name of this type of reaction.
(d) (i) Aqueous bromine was added to a sample of ethene.
Give the colour change seen.
(ii) Explain, in terms of bonding, why there is no colour change when aqueous bromine is added to ethane
(e) There are two structural isomers with the formula \(\mathrm{C}_4 \mathrm{H}_{10}\).
(i) Draw the structures of both of these isomers, showing all of the atoms and all of the bonds.

(ii) Butane is formed when longer chain hydrocarbons are cracked.
Complete the chemical equation to show the other product when butane is formed by cracking.
\[ \mathrm{C}_6 \mathrm{H}_{14} \rightarrow \mathrm{C}_4 \mathrm{H}_{10} + \ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots \ldots . . \cdots \]
(f) A compound contains \(85.7\%\) carbon and \(14.3\%\) hydrogen by mass.
(i) Calculate the empirical formula of this compound.
Show your working.
(ii) The molecular mass of the compound is 112.
Calculate the molecular formula of this compound.
▶️ Answer/Explanation
(a) (i) The homologous series is alkanes.
(a) (ii) Similarities in a homologous series:
1. Same general formula (\(\mathrm{C}_n \mathrm{H}_{2n+2}\) for alkanes).
2. Gradual variation in physical properties (e.g., boiling points increase with chain length).
(b) Number of molecules in 15 g of ethane:
– Molar mass of \(\mathrm{C}_2 \mathrm{H}_6 = 30 \mathrm{~g/mol}\).
– Moles in 15 g = \(\frac{15}{30} = 0.5 \mathrm{~mol}\).
– Molecules = \(0.5 \times 6.02 \times 10^{23} = 3.01 \times 10^{23}\).
(c) (i) Product without carbon: HCl (hydrogen chloride).
(c) (ii) Organic product (1-chloropropane):
(c) (iii) Reaction type: Substitution (chlorine replaces hydrogen).
(d) (i) Colour change: Orange to colourless (bromine reacts with ethene’s double bond).
(d) (ii) No reaction with ethane because it has only single bonds (no π-electrons to react with bromine).
(e) (i) Isomers of \(\mathrm{C}_4 \mathrm{H}_{10}\):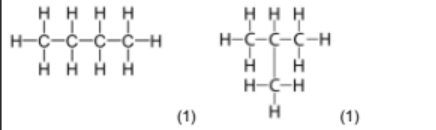
Butane (straight chain) and 2-methylpropane (branched).
(e) (ii) Cracking equation: \(\mathrm{C}_6 \mathrm{H}_{14} \rightarrow \mathrm{C}_4 \mathrm{H}_{10} + \mathrm{C}_2 \mathrm{H}_4\).
(f) (i) Empirical formula:
– Assume 100 g sample: 85.7 g C and 14.3 g H.
– Moles of C = \(\frac{85.7}{12} = 7.14\); moles of H = \(\frac{14.3}{1} = 14.3\).
– Ratio = \(7.14 : 14.3 = 1 : 2\) → \(\mathrm{CH}_2\).
(f) (ii) Molecular formula:
– Empirical mass = 14 g/mol.
– Multiplier = \(\frac{112}{14} = 8\) → \(\mathrm{C}_8 \mathrm{H}_{16}\).
