This question is about hydrocarbons.
(a) State the meaning of the term hydrocarbon.
(b) Propene, \( C_3H_6 \), can be made from long-chain alkanes such as dodecane. Dodecane contains 12 carbon atoms.
(i) Deduce the molecular formula of dodecane.
(ii) Name the type of reaction that occurs when long-chain alkanes are converted into shorter chain alkenes.
(c) Propene is an unsaturated hydrocarbon. Propene reacts with bromine.
(i) State the meaning of the term unsaturated.
(ii) Write the molecular formula of the product formed when propene reacts with bromine.
(d) A styrene molecule is represented as shown in Fig. 6.1.
Fig. 6.1
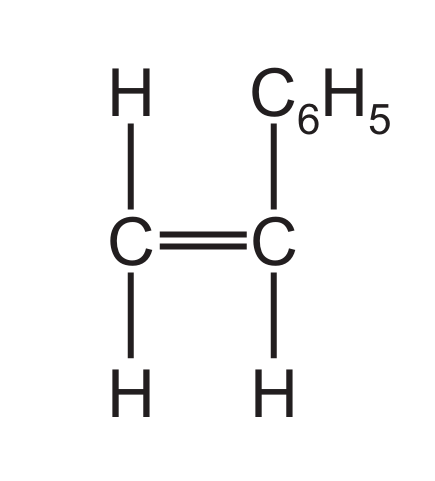
(i) The molecular formula of styrene is \( \text{C}_8\text{H}_8 \). Determine the empirical formula of styrene.
(ii) Styrene can be polymerised into poly(styrene). State the type of polymerisation that occurs when styrene is converted into poly(styrene).
(iii) Draw the structure of one repeat unit of poly(styrene). Include all of the atoms and all of the bonds. The \( \text{C}_8\text{H}_5 \) group should be represented as \( \text{C}_8\text{H}_5 \).
▶️ Answer/Explanation
(a) A compound containing carbon and hydrogen only.
(b)(i) \( C_{12}H_{26} \)
Alkanes have the general formula \( C_nH_{2n+2} \). For 12 carbon atoms (n=12), the number of hydrogen atoms would be \( 2(12)+2 = 26 \).
(b)(ii) Cracking
Cracking is the process where long-chain hydrocarbons are broken down into shorter, more useful molecules including alkenes.
(c)(i) Contains at least one carbon-carbon double bond.
Unsaturated hydrocarbons have double or triple bonds between carbon atoms, allowing them to undergo addition reactions.
(c)(ii) \( C_3H_6Br_2 \)
When propene (\( C_3H_6 \)) reacts with bromine (\( Br_2 \)), the double bond breaks and each carbon forms a bond with a bromine atom, resulting in 1,2-dibromopropane.
(d)(i) \( CH \)
The empirical formula is the simplest ratio of elements. For \( C_8H_8 \), the ratio is 1:1.
(d)(ii) Addition polymerisation
Styrene undergoes addition polymerisation where the double bond opens up to form bonds with adjacent molecules, creating a long polymer chain.
(d)(iii)
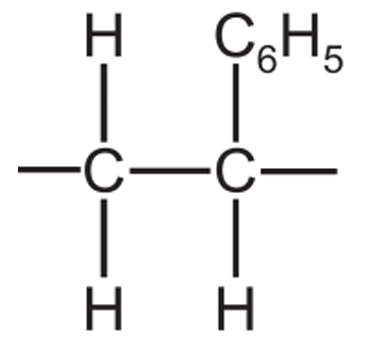
The repeat unit shows the opened double bond with extension bonds at each end, indicating where it connects to other repeat units in the polymer chain.
This question is about alkanes and alkenes.
(a) Short-chain alkanes and alkenes can be formed from long-chain alkanes in a chemical reaction.
(i) Name the type of chemical reaction which forms short-chain alkanes and alkenes from long-chain alkanes.
(ii) Decane has 10 carbon atoms. It forms ethane and ethene as the only products in this type of chemical reaction.
Write the chemical equation for this reaction.
(b) Ethane reacts with chlorine at room temperature to form chloroethane, C2H5Cl, and one other product.
(i) Name the other product formed.
(ii) State the condition needed for this reaction to take place.
(c) Ethene reacts with chlorine at room temperature to form dichloroethane, C2H4Cl2.
C2H4 + Cl2 → C2H4Cl2
(i) State why this is an addition reaction.
(ii) The chemical equation for this reaction can be represented as shown.

The energy change for the reaction is –180kJ/mol.
Use the bond energies in the table to calculate the bond energy of a C–Cl bond, in kJ/mol.

Use the following steps.
step 1 Calculate the energy needed to break bonds.
energy needed to break bonds = kJ
step 2 Use your answer in step 1 and the energy change for the reaction to determine the energy released when bonds are formed.
energy released when bonds form = kJ
step 3 Use your answer in step 2 and bond energy values to determine the energy of a C–Cl bond.
bond energy of a C–Cl bond = kJ/mol
▶️ Answer/Explanation
(a) (i) The reaction is cracking (thermal decomposition of long-chain hydrocarbons).
(a) (ii) Balanced equation for decane cracking: C10H22 → 4C2H4 + C2H6
- Decane (C10H22) breaks into 4 ethene molecules and 1 ethane molecule.
- Note: Mass balance is conserved (10 C and 22 H atoms on both sides).
(b) (i) The other product is hydrogen chloride (HCl) (substitution reaction).
(ii) Condition: UV light (initiates free-radical substitution).
(c) (i) This is an addition reaction because chlorine atoms add across the C=C double bond, forming a single product (dichloroethane).
(c) (ii) Bond energy calculation:
- Energy to break bonds:
- C=C: 610 kJ/mol
- Cl–Cl: 240 kJ/mol
- 4 × C–H: 4 × 410 = 1640 kJ/mol
- Total: 610 + 240 + 1640 = 2490 kJ/mol
- Energy released in bond formation:
- Total energy change = –180 kJ/mol (exothermic)
- Energy released = 2490 + 180 = 2670 kJ/mol
- C–Cl bond energy:
- Energy used for new bonds in C2H4Cl2:
- 4 × C–H: 4 × 410 = 1640 kJ/mol
- C–C: 350 kJ/mol
- Total for C–H and C–C: 1640 + 350 = 1990 kJ/mol
- Energy left for 2 × C–Cl: 2670 – 1990 = 680 kJ/mol
- Per C–Cl bond: 680 ÷ 2 = 340 kJ/mol
- Energy used for new bonds in C2H4Cl2:
