This question is about sodium and compounds of sodium.
(a) (i) Describe the bonding in a metallic element such as sodium.
You may include a diagram as part of your answer.
(ii) Describe how solid sodium conducts electricity.
(b) Some properties of sodium chloride are shown:
- melting point of 801°C
- non-conductor of electricity when solid
- conductor of electricity when molten
- soluble in water.
(i) Name the type of bonding in sodium chloride.
(ii) Explain why sodium chloride conducts electricity when molten.
(c) A student determines the concentration of a solution of dilute sulfuric acid, H2SO4, by titration with aqueous sodium hydroxide, NaOH.
step 1 25.0cm3 of 0.200mol/dm3 NaOH is transferred into a conical flask.
step 2 Three drops of methyl orange indicator are added to the conical flask.
step 3 A burette is filled with H2SO4.
step 4 The acid in the burette is added to the conical flask until the indicator changes colour. The volume of acid is recorded. This process is known as titration.
step 5 The titration is repeated several times until a suitable number of results is obtained.
(i) Name the piece of apparatus used to measure exactly 25.0cm3 of 0.200mol/dm3 NaOH in step 1.
(ii) State the colour change of the methyl orange indicator in step 4.
(iii) State how the student decides that a suitable number of results have been obtained.
(iv) 20.0cm3 of H2SO4 reacts with 25.0cm3 of 0.200mol/dm3 NaOH.
The equation for the reaction is shown.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Calculate the concentration of H2SO4 using the following steps.
- Calculate the number of moles in 25.0cm3 of 0.200mol/dm3 NaOH.
mol
- Determine the number of moles of H2SO4 that react with the NaOH.
mol
- Calculate the concentration of H2SO4.
mol/dm3
▶️ Answer/Explanation
(a) (i) Metallic bonding consists of a lattice of positive sodium ions (\( \text{Na}^+ \)) surrounded by a “sea” of delocalized electrons. The electrostatic attraction between the cations and electrons holds the structure together. (Diagram: ions in a fixed arrangement with overlapping electron clouds.)
(a) (ii) Solid sodium conducts electricity because its delocalized electrons are free to move and carry charge.
(b) (i) The bonding in sodium chloride is ionic, involving electrostatic attraction between \( \text{Na}^+ \) and \( \text{Cl}^- \) ions.
(b) (ii) Molten sodium chloride conducts electricity because the ions (\( \text{Na}^+ \) and \( \text{Cl}^- \)) become mobile and can carry charge.
(c) (i) The apparatus is a pipette, which precisely measures 25.0 cm³ of NaOH.
(c) (ii) Methyl orange changes from yellow (alkaline) to orange (neutral) at the endpoint.
(c) (iii) A suitable number of results is achieved when at least two titrations agree within 0.2 cm³ (concordant results).
(c) (iv)
- Moles of NaOH: \( \frac{25.0}{1000} \times 0.200 = 0.005 \) mol.
- Moles of H2SO4: \( \frac{0.005}{2} = 0.0025 \) mol (1:2 stoichiometry).
- Concentration of H2SO4: \( \frac{0.0025 \times 1000}{20.0} = 0.125 \) mol/dm³.
A teacher demonstrated the reactivity of calcium with water. He used the apparatus shown below.
(a) The teacher measured the volume of gas given off at various times during the reaction. He then repeated the experiment using strontium but keeping all the conditions the same. The graph obtained from the results is shown below.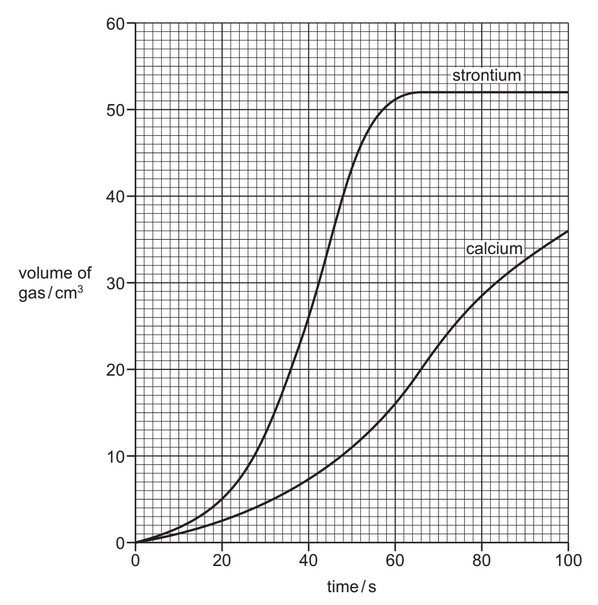
(i) Explain how the graph shows that strontium is more reactive than calcium.
(ii) For the reaction between calcium and water, deduce the volume of gas produced in the first 50 seconds.
(iii) At what time was the reaction between strontium and water complete?
(iv) How do you know from the graph that the reaction between calcium and water was not complete 100 seconds after the reaction started?
(v) Suggest how the rate of reaction changes when the same mass of calcium is used but in smaller pieces.
(b) The solution formed at the end of the reaction between strontium and water is alkaline. It is a solution of strontium hydroxide.
The teacher titrated this solution with hydrochloric acid using the apparatus shown below.
(i) What piece of apparatus should be used to put exactly 25.0 cm3 of the strontium hydroxide solution into the flask?
(ii) A few drops of litmus solution was added to the flask. Explain why litmus is added to the flask and describe what happens to the litmus as the titration proceeds.
(c) The graph below shows how the pH of the solution in the flask changes as the acid is added.
(i) Describe how the pH of the solution changes as the titration proceeds.
(ii) What volume of acid had been added when the solution had a neutral pH?
(iii) The symbol equation for the reaction is \( Sr(OH)_2 + 2HCl \rightarrow SrCl_2 + 2H_2O \). Give the name of the salt formed in this reaction.
▶️ Answer/Explanation
(a)(i) The steeper slope of the strontium curve indicates a faster reaction rate, proving strontium is more reactive than calcium.
(a)(ii) From the graph, the volume of gas produced by calcium in 50 seconds is 11 cm3.
(a)(iii) The reaction completes at 65 seconds (where the strontium curve plateaus).
(a)(iv) The calcium curve was still rising at 100 seconds, indicating the reaction was incomplete.
(a)(v) Smaller calcium pieces increase the surface area, thus increasing the reaction rate.
(b)(i) A volumetric pipette measures exactly 25.0 cm3 of the solution.
(b)(ii) Litmus acts as a pH indicator: it changes from blue (alkaline) to pink (acidic) at the endpoint.
(c)(i) pH decreases slowly initially, drops sharply near the equivalence point (26 cm3), then stabilizes.
(c)(ii) Neutral pH occurs at 26 cm3 of acid added.
(c)(iii) The salt formed is strontium chloride (SrCl2).
