(a) Aqueous ammonium sulfate, \((\mathrm{NH}_4)_2\mathrm{SO}_4\), is warmed with aqueous sodium hydroxide. The pungent-smelling gas ammonia, \(\mathrm{NH}_3\), is produced. Balance the equation for this reaction.
\( (\mathrm{NH}_4)_2\mathrm{SO}_4 + \ldots \mathrm{NaOH} \rightarrow \ldots \mathrm{NH}_3 + \ldots \mathrm{H}_2\mathrm{O} + \mathrm{Na}_2\mathrm{SO}_4 \)
(b) A \(2.8 \mathrm{~g}\) sample of impure ammonium sulfate is found to contain \(0.7 \mathrm{~g}\) of impurities. Calculate the percentage of ammonium sulfate in this sample.
(c) Describe a test for ammonia gas.
(d) Ammonia gas is prepared at the front of a laboratory. The pungent smell of ammonia spreads throughout the laboratory slowly.
(i) Name the process that occurs when ammonia gas spreads throughout the laboratory.
(ii) Explain, using ideas about particles, why ammonia gas spreads throughout the laboratory.
(iii) Explain why carbon dioxide gas, \(\mathrm{CO}_2\), will spread throughout the laboratory at a slower rate than ammonia gas, \(\mathrm{NH}_3\).
(e) Ammonia is produced in the Haber process. The equation for the reaction is shown.
\( \mathrm{N}_2(\mathrm{g}) + 3\mathrm{H}_2(\mathrm{g}) \rightarrow 2\mathrm{NH}_3(\mathrm{g}) \)
(i) In the Haber process, a temperature of \(450^\circ \mathrm{C}\) and a pressure of 200 atmospheres are used in the presence of finely-divided iron. A larger equilibrium yield of ammonia would be produced if a lower temperature and a higher pressure are used. Explain why a lower temperature and a higher pressure are not used.
(ii) State the role of iron in the Haber process.
(f) Ammonia is a weak base.
(i) Explain the meaning of the term base.
(ii) Suggest the pH of aqueous ammonia.
▶️ Answer/Explanation
(a) The balanced equation is:
\( (\mathrm{NH}_4)_2\mathrm{SO}_4 + 2\mathrm{NaOH} \rightarrow 2\mathrm{NH}_3 + 2\mathrm{H}_2\mathrm{O} + \mathrm{Na}_2\mathrm{SO}_4 \)
(b) Percentage purity = \(\frac{2.8 – 0.7}{2.8} \times 100 = 75\%\).
(c) Test: Damp red litmus paper.
Result: Turns blue (ammonia is alkaline).
(d)(i) The process is diffusion.
(d)(ii) Ammonia particles move randomly from high to low concentration until evenly distributed.
(d)(iii) \(\mathrm{CO}_2\) diffuses slower because its molecules are heavier (44 g/mol vs. 17 g/mol for \(\mathrm{NH}_3\)).
(e)(i) Lower temperature: Slows reaction rate, reducing ammonia production.
Higher pressure: Requires expensive, high-strength equipment.
(e)(ii) Iron acts as a catalyst to speed up the reaction.
(f)(i) A base accepts protons (H⁺ ions) or donates electron pairs.
(f)(ii) Aqueous ammonia has a pH of 10–11 (weakly alkaline).
The diagram shows a bottle of mineral water. The concentration of the ions present in the water is shown on the label. The pH of the water is also shown.
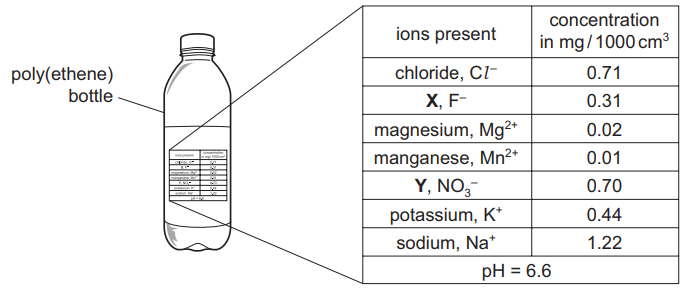
(a) (i) Which positively charged ion is present in the highest concentration? [1]
(ii) State the name of:
ion X
ion Y
(iii) Calculate the mass, in mg, of sodium ions in 200 cm3 of mineral water.
(iv) Which one of the following phrases best describes the pH of this mineral water? Tick one box.

(b) Describe a test for chloride ions.
(c) The mineral water bottle is made of poly(ethene).
Complete the following sentence about poly(ethene) using words from the list below.
atom ionic monomer polymer reactant saturated
Poly(ethene) is a ___ made by the addition of ___ units.
▶️ Answer/Explanation
(a)(i) The highest concentration positively charged ion is sodium (Na+) (1.22 mg/L).
(a)(ii) Identification:
- X: Fluoride (F–)
- Y: Nitrate (NO3–)
(a)(iii) Mass of sodium ions:
- Concentration: 1.22 mg/L.
- Volume: 200 cm3 = 0.2 L.
- Mass = 1.22 mg/L × 0.2 L = 0.244 mg (accept 0.24 mg).
(a)(iv) The pH (6.5) indicates the water is weakly acidic (4th box ticked).
(b) Chloride ion test:
- Test: Add dilute nitric acid followed by silver nitrate solution.
- Result: White precipitate (AgCl) forms.
(c) Poly(ethene) is a polymer made by the addition of monomer units (ethene molecules).
