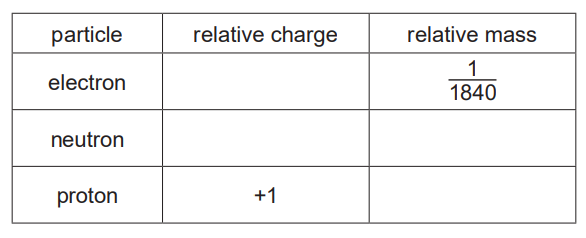
(a) Atoms are made of protons, neutrons and electrons. Atoms of the same element are known as isotopes.
(i) Complete the table.
(ii) \(_{12}^{24}\textrm{Mg}\) and \(_{12}^{25}\textrm{Mg}\) are isotopes of magnesium.
Complete the table to show the numbers of electrons, neutrons and protons in these isotopes of magnesium.

(iii) Explain why magnesium ions have a charge of 2+.
(b) Mg2+ ions have the electronic structure 2,8.
Give the formula of the following particles which have the same electronic structure as Mg2+ ions.
- a cation (positive ion)
- an anion (negative ion)
- an atom
▶️ Answer/Explanation
2(a)(i)
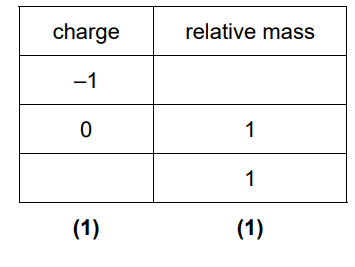
Mark by column
2(a)(ii)

Mark by row
2(a)(iii) (they have) 2 more protons than electrons
OR
(they have) 2 fewer electrons than protons
OR
(they have) 12 protons and 10 electrons
2(b) Na+ or Al3+ (1)
F– or O2– or N3– (1)
Ne (1)
Detailed Solution:
(a)(i) The table is completed by identifying the subatomic particles: protons (positive), neutrons (neutral), and electrons (negative). Their relative masses are 1, 1, and ~1/1836 respectively.
(a)(ii) For \(_{12}^{24}\textrm{Mg}\) and \(_{12}^{25}\textrm{Mg}\):
- Both have 12 protons (atomic number) and 12 electrons (neutral atoms).
- Neutrons are calculated as mass number minus protons: 12 for \(_{12}^{24}\textrm{Mg}\) and 13 for \(_{12}^{25}\textrm{Mg}\).
(a)(iii) Mg2+ ions lose 2 electrons, leaving 10 electrons vs. 12 protons, resulting in a 2+ charge.
(b) Particles with the same electronic structure (2,8) as Mg2+:
- Cation: Na+ (loses 1 electron) or Al3+ (loses 3 electrons).
- Anion: F– (gains 1 electron), O2– (gains 2 electrons), or N3– (gains 3 electrons).
- Atom: Neon (Ne), a noble gas with a full outer shell.
This question is about copper and its compounds.
(a) Copper has two different naturally occurring atoms, \(^{63}Cu\) and \(^{65}Cu\).
(i) State the term used for atoms of the same element with different nucleon numbers.
(ii) The atomic number of copper is 29. Complete the table to show the number of protons, neutrons and electrons in the particles of copper shown.

(iii) Relative atomic mass is the average mass of naturally occurring atoms of an element. The percentage of the naturally occurring atoms in a sample of copper is shown.

Deduce the relative atomic mass of copper in this sample. Give your answer to one decimal place.
(b) Anhydrous copper(II) sulfate is used to test for the presence of water. When this test is positive, hydrated copper(II) sulfate is formed.
(i) State the colour change seen during this test.
(ii) Complete the chemical equation to show the reaction that takes place.
\(CuSO_4 + …………………… \leftrightarrow CuSO_4•5H_2O\)
(iii) State how hydrated copper(II) sulfate can be turned back into anhydrous copper(II) sulfate.
(iv) Describe a test for pure water.
(c) Aqueous copper(II) sulfate contains \(Cu^{2+}\)(aq) ions.
(i) Describe what is seen when aqueous copper(II) sulfate is added to aqueous sodium hydroxide, NaOH(aq).
(ii) Write the ionic equation for the reaction between aqueous copper(II) sulfate and aqueous sodium hydroxide. Include state symbols.
(d) When solid copper(II) nitrate is heated copper(II) oxide, nitrogen dioxide and oxygen are formed.
\(2Cu(NO_3)_2 → 2CuO + 4NO_2 + O_2\)
Calculate the volume of nitrogen dioxide formed at room temperature and pressure when 4.7g of \(Cu(NO_3)_2\) is heated. Use the following steps:
- calculate the mass of one mole of \(Cu(NO_3)_2\)
- calculate the number of moles of \(Cu(NO_3)_2\) used
- determine the number of moles of nitrogen dioxide formed
- calculate the volume of nitrogen dioxide formed at room temperature and pressure.
(e) Write the chemical equation to show the action of heat on sodium nitrate, \(NaNO_3\).
▶️ Answer/Explanation
(a) (i) Ans: isotopes
Atoms of the same element with different nucleon numbers are called isotopes.
(ii) Ans:

For \(^{63}Cu\): 29 protons, 34 neutrons, 29 electrons. For \(^{65}Cu\): 29 protons, 36 neutrons, 29 electrons.
(iii) Ans: 63.6
Relative atomic mass = \((70 \times 63 + 30 \times 65) / 100 = 63.6\).
(b) (i) Ans: white to blue
Anhydrous copper(II) sulfate is white, and it turns blue when hydrated.
(ii) Ans: \(5H_2O\)
The reaction is \(CuSO_4 + 5H_2O \leftrightarrow CuSO_4•5H_2O\).
(iii) Ans: heating
Heating removes water molecules, converting hydrated copper(II) sulfate back to anhydrous form.
(iv) Ans: Boiling point is 100°C or freezing point is 0°C
Pure water boils at 100°C or freezes at 0°C under standard conditions.
(c) (i) Ans: blue precipitate
A blue precipitate of copper(II) hydroxide forms when the solutions are mixed.
(ii) Ans: \(Cu^{2+}(aq) + 2OH^-(aq) \rightarrow Cu(OH)_2(s)\)
The ionic equation shows the formation of solid copper(II) hydroxide.
(d) Ans: 1.2 dm³
Molar mass of \(Cu(NO_3)_2\) = 187.5 g/mol. Moles of \(Cu(NO_3)_2\) = 4.7/187.5 ≈ 0.025. Moles of \(NO_2\) = 0.05. Volume = 0.05 × 24 = 1.2 dm³.
(e) Ans: \(2NaNO_3 \rightarrow 2NaNO_2 + O_2\)
Heating sodium nitrate produces sodium nitrite and oxygen gas.
