This question is about compounds of tin.
(a) Tin(IV) oxide has the formula SnO₂. The relative formula mass, \( M_r \) of SnO₂ is 151. Calculate the percentage by mass of tin in SnO₂.
(b) SnO₂ is an amphoteric oxide. SnO₂ reacts with aqueous sodium hydroxide, NaOH, to form a sodium salt and water only. The sodium salt contains a negative ion with the formula SnO₃²⁻.
(i) State the meaning of the term amphoteric.
(ii) Write the symbol equation for the reaction between SnO₂ and NaOH.
(c) Tin is a metal that forms both covalent and ionic compounds. Suggest why this is unusual for a metal.
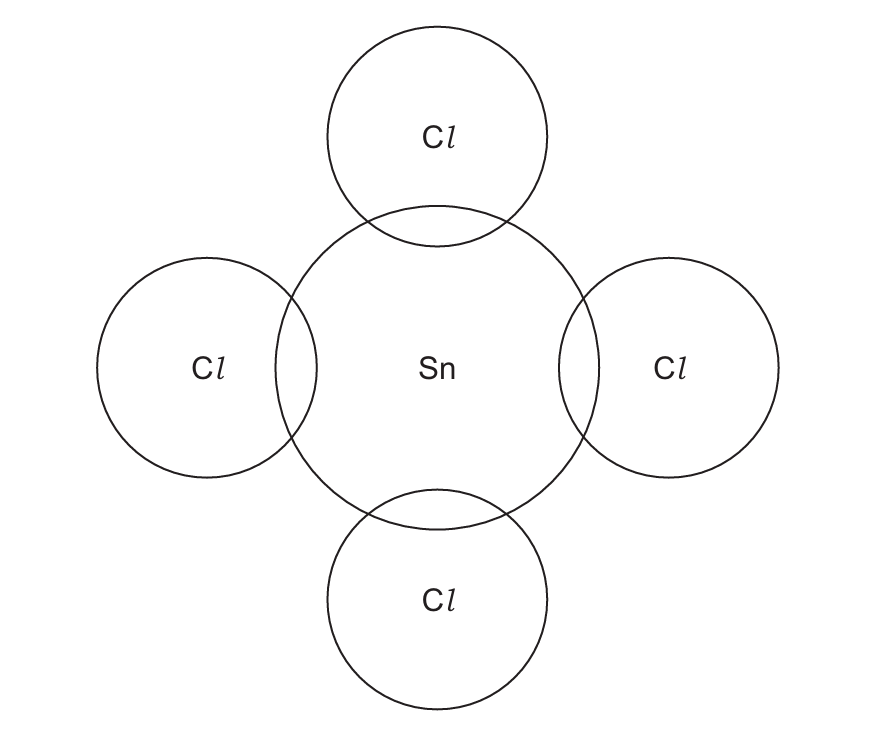
(d) (i) Tin(IV) chloride, SnCl₄ is covalently bonded. A tin atom has four electrons in its outer shell. Complete the dot-and-cross diagram in Fig. 3.1 for a molecule of SnCl₄. Show the outer shell electrons only.
(ii) Tin(II) oxide, SnO, is ionically bonded. The melting points of SnCl₄ and SnO are shown in Table 3.1.
Table 3.1

Explain, in terms of structure and bonding, why SnCl₄ has a much lower melting point than SnO.
(e) Part of the reactivity series is shown.
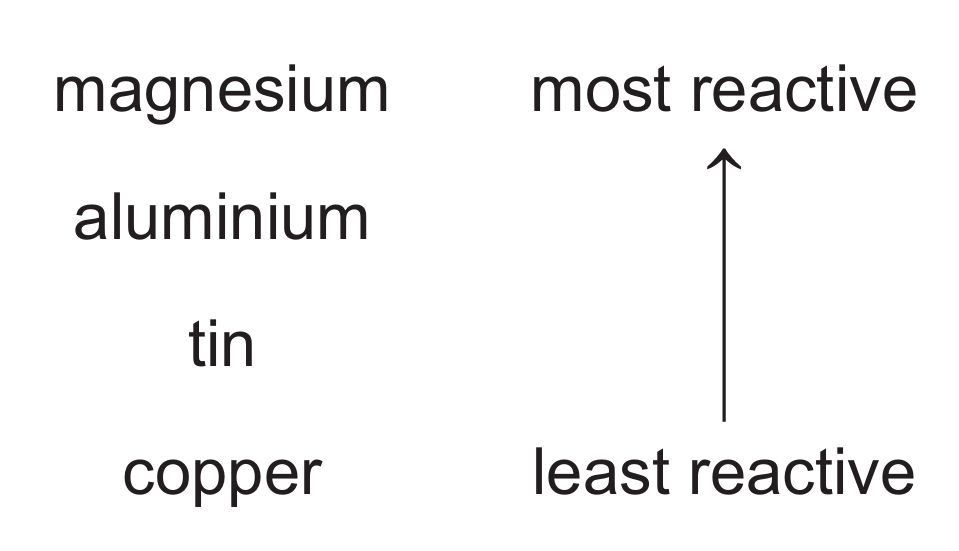
(i) When aluminium foil is added to aqueous tin(II) sulfate, a reaction does not occur even though aluminium is above tin in the reactivity series. Explain why a reaction does not occur.
(ii) An aqueous solution of tin(II) sulfate contains Sn²⁺ ions. Two experiments are carried out.
Experiment 1 Copper is added to aqueous tin(II) sulfate.
Experiment 2 Magnesium is added to aqueous tin(II) sulfate.
Write an ionic equation for any reaction that occurs in each experiment. If no reaction occurs, write ‘no reaction’.
(f) Hydrated tin(II) nitrate, Sn(NO₃)₂•20H₂O, decomposes when it is heated.
(i) State what is meant by the term hydrated.
(ii) Complete the equation for the decomposition of Sn(NO₃)₂•20H₂O.
2Sn(NO₃)₂•20H₂O → ……SnO + ……NO₂ + O₂ + ……H₂O
▶️ Answer/Explanation
(a) 78.8%
Calculation: Relative atomic mass of Sn = 119, O = 16. Percentage of Sn = (119/151) × 100 = 78.8%.
(b)(i) Reacts with acids and with bases to produce a salt and water.
(b)(ii) \( \text{SnO}_2 + 2\text{NaOH} \rightarrow \text{Na}_2\text{SnO}_3 + \text{H}_2\text{O} \)
The reaction shows tin(IV) oxide reacting with sodium hydroxide to form sodium stannate (Na₂SnO₃) and water.
(c) Metals typically form ionic compounds only, while covalent compounds usually contain non-metals only. Tin’s ability to form both is unusual.
(d)(i) The dot-and-cross diagram should show:
- Four single bonds between tin and chlorine atoms
- Three lone pairs on each chlorine atom
- No lone pairs on the tin atom
(d)(ii)
- SnCl₄ has simple molecular structure with weak intermolecular forces between molecules
- SnO has giant ionic structure with strong electrostatic forces between ions
- Much more energy is needed to overcome the strong ionic bonds in SnO than the weak intermolecular forces in SnCl₄
(e)(i) Aluminium forms an unreactive coating of aluminium oxide that prevents reaction.
(e)(ii)
- Experiment 1: no reaction (copper is below tin in reactivity series)
- Experiment 2: \( \text{Mg} + \text{Sn}^{2+} \rightarrow \text{Mg}^{2+} + \text{Sn} \)
(f)(i) A substance that is chemically combined with water or contains water of crystallization.
(f)(ii) \( 2\text{Sn(NO}_3\text{)}_2•20\text{H}_2\text{O} \rightarrow 2\text{SnO} + 4\text{NO}_2 + \text{O}_2 + 40\text{H}_2\text{O} \)
Balancing: For every 2 formula units of the hydrated salt, we get 2 SnO, 4 NO₂ (from 4 nitrate groups), 1 O₂ (remaining oxygen), and 40 H₂O (from 20 × 2 water molecules).
Potassium reacts with chlorine to form potassium chloride, KCl.
(a) Write a chemical equation for this reaction.
(b) Potassium chloride is an ionic compound. Complete the diagram to show the electron arrangement in the outer shells of the ions present in potassium chloride. Give the charges on both ions.

(c) Molten potassium chloride undergoes electrolysis.
(i) State what is meant by the term electrolysis.
(ii) Name the products formed at the positive electrode (anode) and negative electrode (cathode) when molten potassium chloride undergoes electrolysis.
(d) Concentrated aqueous potassium chloride undergoes electrolysis.
(i) Write an ionic half-equation for the reaction at the negative electrode (cathode).
(ii) Name the product formed at the positive electrode (anode).
(iii) Name the potassium compound that remains in the solution after electrolysis.
(e) Complete the dot-and-cross diagram to show the electron arrangement in a molecule of chlorine, \(Cl_2\). Show the outer electrons only.

(f) The melting points and boiling points of chlorine and potassium chloride are shown.

(i) Deduce the physical state of chlorine at –75°C. Use the data in the table to explain your answer.
(ii) Explain, in terms of structure and bonding, why potassium chloride has a much higher melting point than chlorine.
Your answer should refer to the:
- types of particle held together by the forces of attraction
- types of forces of attraction between particles
- relative strength of the forces of attraction.
▶️ Answer/Explanation
(a) \(2K + Cl_2 → 2KCl\)
Explanation: Potassium (K) reacts with chlorine gas (\(Cl_2\)) to form potassium chloride (KCl). The equation is balanced with coefficients to ensure conservation of mass.
(b) K outer shell with 8 crosses (1), Cl outer shell with 7 dots and 1 cross (1), \(^+\) and – (1)
Explanation: Potassium loses 1 electron (forming \(K^+\)) and chlorine gains 1 electron (forming \(Cl^-\)). The diagram should show \(K^+\) with an empty outer shell and \(Cl^-\) with a full outer shell (8 electrons).
(c)(i) Breakdown by (the passage of) electricity (1) of an ionic compound in molten or aqueous (state) (1)
Explanation: Electrolysis is the decomposition of an ionic compound into its elements using electricity.
(c)(ii) (anode) chlorine, (cathode) potassium
Explanation: In molten KCl, \(Cl^-\) ions are oxidized to \(Cl_2\) at the anode, while \(K^+\) ions are reduced to K at the cathode.
(d)(i) \(2H^+ + 2e^– → H_2\)
Explanation: In aqueous KCl, water is reduced instead of \(K^+\), producing hydrogen gas at the cathode.
(d)(ii) chlorine
Explanation: \(Cl^-\) ions are still oxidized to \(Cl_2\) at the anode in aqueous solution.
(d)(iii) potassium hydroxide (1)
Explanation: The remaining solution contains \(K^+\) and \(OH^-\) ions, forming KOH.
(e) One shared pair of electrons and 6 non-bonding electrons on each chlorine atom.
Explanation: A \(Cl_2\) molecule has a single covalent bond (shared pair) and 3 lone pairs (6 electrons) on each Cl atom.
(f)(i) liquid (1)
Explanation: At –75°C, chlorine is between its melting point (–101°C) and boiling point (–35°C), so it exists as a liquid.
(f)(ii) Ionic bonds in KCl (1), attraction between molecules in \(Cl_2\) (1), weaker attraction (between particles) in \(Cl_2\) (1)
Explanation: KCl has strong ionic bonds between ions, requiring high energy to break. \(Cl_2\) has weak van der Waals forces between molecules, requiring less energy.
