The formulae of the molecules A to I are shown in Table 1.1.
Table 1.1
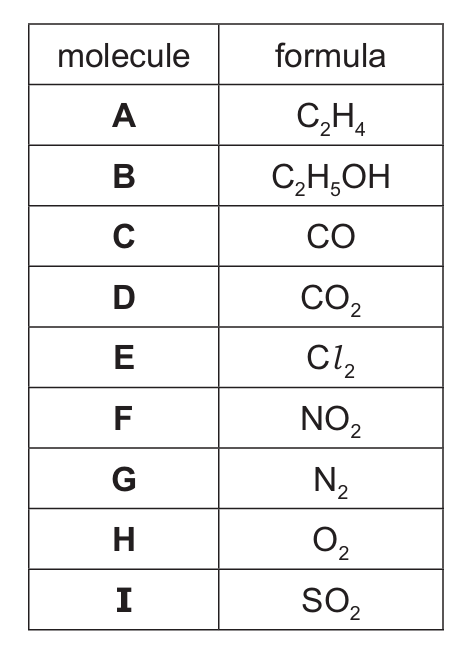
Answer the following questions about the molecules, A to I. Each letter may be used once, more than once or not at all.
State which of the molecules A to I:
(a) is an element with a triple bond
(b) is a product of photosynthesis
(c) is used as a fuel
(d) turns limewater milky
(e) undergoes a substitution reaction with alkanes
(f) is a colourless liquid at r.t.p.
(g) is unsaturated
(h) is 21% of clean, dry air
(i) is a reactant in the Haber process.
▶️ Answer/Explanation
(a) G (\( N_2 \))
Nitrogen (\( N_2 \)) is an element with a triple bond between the two nitrogen atoms.
(b) D (\( CO_2 \))
Carbon dioxide is a product of photosynthesis, along with oxygen.
(c) B (\( C_2H_5OH \))
Ethanol (\( C_2H_5OH \)) is commonly used as a fuel, especially in biofuel applications.
(d) D (\( CO_2 \))
Carbon dioxide turns limewater milky due to the formation of calcium carbonate precipitate.
(e) E (\( Cl_2 \))
Chlorine undergoes substitution reactions with alkanes, replacing hydrogen atoms.
(f) B (\( C_2H_5OH \))
Ethanol is a colourless liquid at room temperature and pressure (r.t.p.).
(g) A (\( C_2H_4 \))
Ethene (\( C_2H_4 \)) is unsaturated as it contains a carbon-carbon double bond.
(h) H (\( O_2 \))
Oxygen makes up approximately 21% of clean, dry air.
(i) G (\( N_2 \))
Nitrogen is one of the reactants in the Haber process for ammonia production.
Sulfur forms two chlorides, P and Q. Chloride P has the formula \( S_2Cl_2 \). Chloride Q has the formula \( SCl_2 \).
(a) Both chlorides are covalently bonded and have low melting points.
Suggest, in terms of attraction between particles, why these chlorides have low melting points.
(b) Chloride P, \( S_2Cl_2 \), forms when sulfur reacts with chlorine.
Write the symbol equation for this reaction.
(c) Complete the dot-and-cross diagram in Fig. 3.1 of a molecule of chloride Q, \( SCl_2 \).
Show outer electrons only.

(d) Chloride P is converted to chloride Q by reaction with chlorine in a closed system. The reversible reaction reaches an equilibrium.
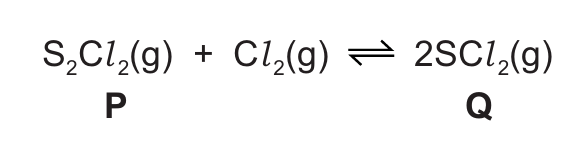
The forward reaction is exothermic.
Suggest two changes to the conditions which will result in a decrease in the concentration of chloride Q at equilibrium.
(e) The rate of the forward reaction in (d) is determined by collision theory.
The rate of reaction depends upon two factors:
- the frequency of collisions between particles
- the proportion of collisions which have energy greater than or equal to the activation energy.
(i) Define the term activation energy.
(ii) Give the symbol for activation energy.
(iii) Complete Table 3.1 to show the effect, if any, when the conditions are changed.
Use only the words increases, decreases or no change.

(f) The reaction of chloride P with chlorine is a redox reaction.
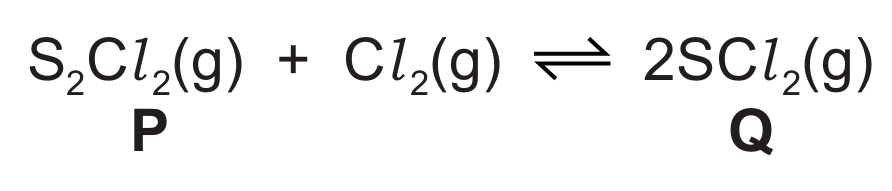
The oxidation number of Cl in chloride P and chloride Q is -1.
Use oxidation numbers to explain why:
- sulfur is oxidised in the forward reaction
- chlorine is oxidised in the reverse reaction.
▶️ Answer/Explanation
(a) These chlorides have low melting points because:
- The attraction between molecules is weak (intermolecular forces)
- They are simple covalent molecules with weak van der Waals’ forces between molecules
(b) The symbol equation is: \( 2S + Cl_2 \rightarrow S_2Cl_2 \)
(c) The dot-and-cross diagram for \( SCl_2 \) should show:
- Sulfur with one bonding pair (dot-cross) with each chlorine
- Sulfur with four non-bonding electrons (dots)
- Each chlorine with six non-bonding electrons (crosses)
(d) To decrease concentration of Q at equilibrium:
- Decrease concentration of \( S_2Cl_2 \) (P) and/or \( Cl_2 \)
- Increase temperature (since forward reaction is exothermic)
(e)(i) Activation energy is the minimum energy that colliding particles must have to react.
(e)(ii) The symbol for activation energy is \( E_a \).
(e)(iii) Table completion:
- Increasing chlorine concentration: increases frequency of collisions, no change to proportion with sufficient energy
- Increasing temperature: increases both frequency and proportion with sufficient energy
- Adding a catalyst: no change to frequency, increases proportion with sufficient energy (by lowering \( E_a \))
(f) Redox explanation:
- Sulfur is oxidized in forward reaction because its oxidation number increases from +1 in \( S_2Cl_2 \) to +2 in \( SCl_2 \)
- Chlorine is oxidized in reverse reaction because its oxidation number increases from -1 in \( SCl_2 \) to 0 in \( Cl_2 \)
In the forward reaction, sulfur’s oxidation state increases (oxidation) while chlorine’s decreases (reduction). The reverse is true for the reverse reaction.
