Aluminium is manufactured by the electrolysis of aluminium oxide.
(a) State the name of the main ore of aluminium.
(b) Name the substance mixed with aluminium oxide to reduce the operating temperature of the process.
(c) Explain why the molten mixture in (b) conducts electricity.
(d) Table 2.1 contains some information about the processes which take place at the anode and the cathode.
Table 2.1
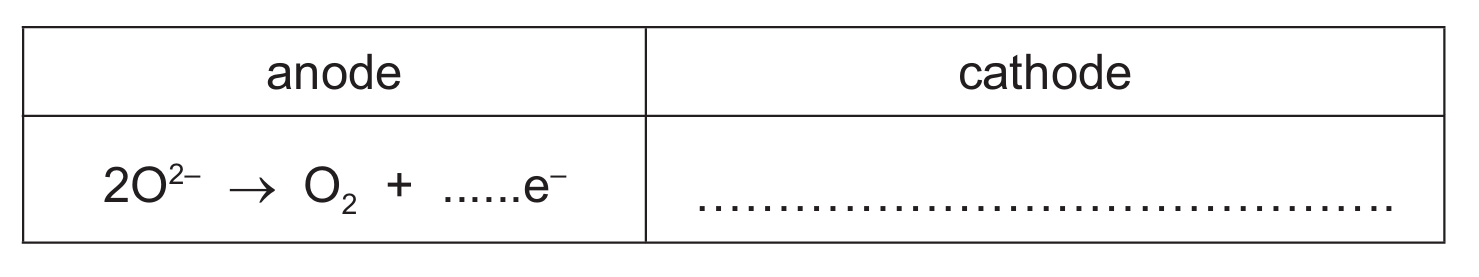
(i) Complete Table 2.1:
– Write the number of electrons needed to balance the ionic half-equation for the reaction at the anode.
– Write the ionic half-equation for the reaction at the cathode.
(ii) State why the process at the anode is an oxidation.
(iii) Oxygen is formed at the anode.
Explain why the main gas given off at the anode is carbon dioxide and not oxygen.
(e) State why aluminium is used in food containers.
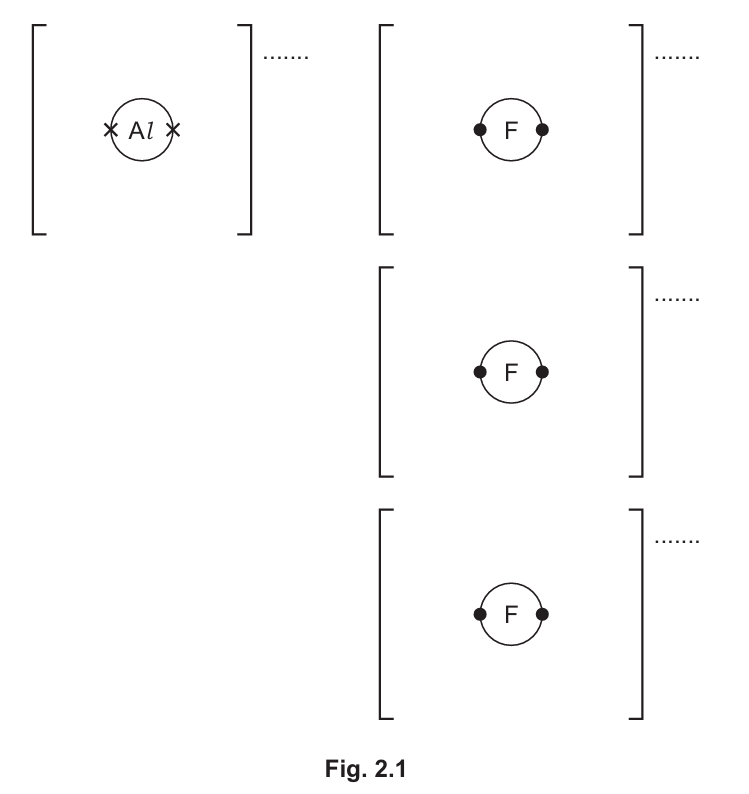
(f) Aluminium reacts with fluorine to form the ionic compound aluminium fluoride.
Complete the dot-and-cross diagram in Fig. 2.1 of the ions in aluminium fluoride.
Give the charges on the ions.
▶️ Answer/Explanation
(a) bauxite
Bauxite is the primary ore from which aluminium is extracted, containing aluminium oxide along with various impurities.
(b) cryolite
Cryolite (Na3AlF6) is mixed with aluminium oxide to lower its melting point from about 2072°C to around 950°C, making the electrolysis process more energy-efficient.
(c) (it has) mobile ions
The molten mixture contains free-moving ions (Al3+ and O2-) which can carry electric charge through the solution, enabling electrical conduction.
(d)(i)
Anode: 4 electrons (\( 2O^{2-} \rightarrow O_2 + 4e^- \))
Cathode: \( Al^{3+} + 3e^- \rightarrow Al \)
The anode equation shows oxygen ions losing electrons to form oxygen gas. The cathode equation shows aluminium ions gaining electrons to form aluminium metal.
(d)(ii) electrons are lost (from oxide ions)
Oxidation is defined as the loss of electrons. At the anode, oxide ions (O2-) lose electrons to form oxygen gas.
(d)(iii)
1. The anode is made of carbon/graphite
2. The carbon anode reacts with the oxygen produced, forming carbon dioxide
Instead of oxygen gas being released, it reacts with the carbon electrodes to form CO2, which is why we observe carbon dioxide rather than oxygen at the anode.
(e) aluminium is resistant to corrosion
Aluminium forms a protective oxide layer that prevents further reaction, making it ideal for food containers where corrosion resistance is important.
(f)
Dot-and-cross diagram should show:
– Aluminium ion (Al3+) with empty outer shell
– Three fluoride ions (F–) each with 8 electrons (7 dots and 1 cross)
Charges: Al3+ and F–
Aluminium fluoride is ionic with Al transferring 3 electrons (one to each F atom) to form Al3+ and F– ions. The diagram should clearly show this electron transfer.
This question is about electrolysis.
(a) State the meaning of the term electrolysis.
(b) Table 2.1 gives some information about the electrolysis of two electrolytes using graphite electrodes.
Table 2.1
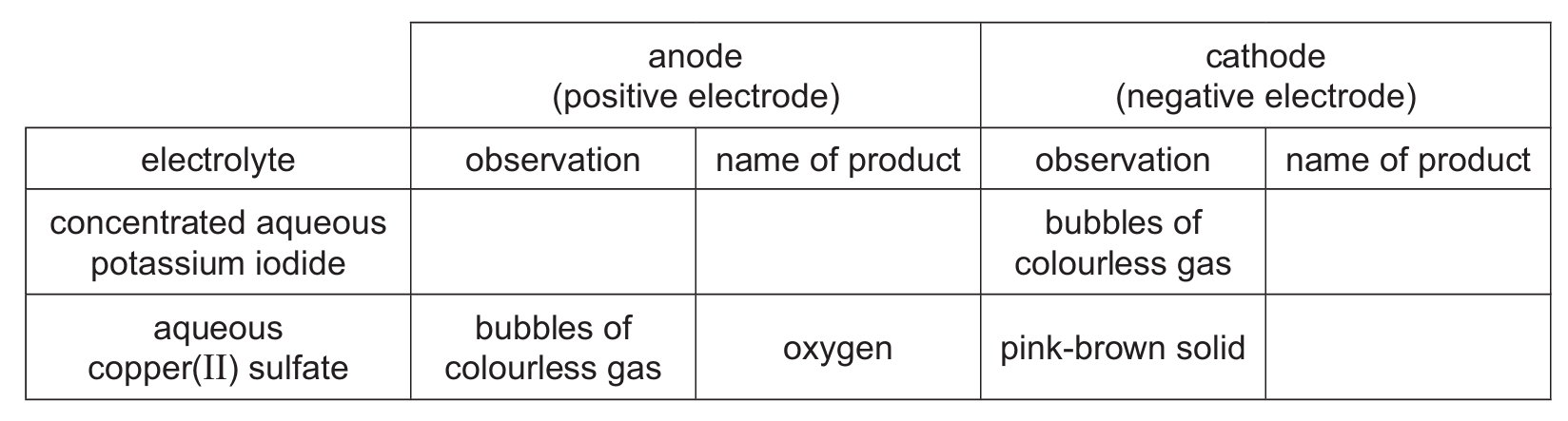
(i) Complete Table 2.1.
(ii) Oxygen is produced at the anode by the electrolysis of aqueous copper(II) sulfate. Write the ionic half-equation for this reaction.
(c) Aqueous copper(II) sulfate is electrolysed using copper electrodes instead of graphite electrodes.
(i) Explain why the mass of the anode decreases during this electrolysis.
(ii) Name the product formed at the cathode.
(iii) State what change, if any, is observed in the appearance of the aqueous copper(II) sulfate.
▶️ Answer/Explanation
(a) Electrolysis is the breakdown of an ionic compound in molten or aqueous state by the passage of electricity.
(b)(i) For concentrated aqueous potassium iodide:
Anode product: iodine (brown solution or black solid)
Cathode product: hydrogen (bubbles of colourless gas)
For aqueous copper(II) sulfate:
Cathode product: copper (pink-brown solid)
(b)(ii) The ionic half-equation for oxygen production at the anode is:
\(4OH^- \rightarrow 2H_2O + O_2 + 4e^-\)
(c)(i) The mass of the anode decreases because copper atoms from the anode lose electrons to form copper ions (\(Cu^{2+}\)) which go into the solution.
(c)(ii) The product formed at the cathode is copper.
(c)(iii) There is no change in the appearance of the aqueous copper(II) sulfate solution.
Detailed Explanation:
1. Electrolysis definition: Electrolysis involves the decomposition of ionic compounds when electricity is passed through them in molten or aqueous state. The ions move to the oppositely charged electrodes where they gain or lose electrons.
2. Electrolysis of potassium iodide:
– At the anode: Iodide ions (\(I^-\)) are oxidized to iodine (\(I_2\)), which appears as a brown solution or black solid.
– At the cathode: Hydrogen ions (\(H^+\)) from water are reduced to hydrogen gas (\(H_2\)), seen as bubbles.
3. Electrolysis of copper(II) sulfate:
– At the anode: Hydroxide ions (\(OH^-\)) from water are oxidized to oxygen gas (\(O_2\)) and water.
– At the cathode: Copper ions (\(Cu^{2+}\)) are reduced to copper metal, forming a pink-brown deposit.
4. Using copper electrodes:
– The copper anode dissolves as copper atoms lose electrons to become \(Cu^{2+}\) ions.
– At the cathode, these \(Cu^{2+}\) ions are reduced back to copper metal.
– The concentration of copper ions in solution remains constant, so no color change occurs.
