This question is about rate of reaction and equilibrium.
A student investigates the rate of decomposition of aqueous hydrogen peroxide, \( H_2O_2 \), using manganese(IV) oxide as a catalyst.
The equation for the reaction is shown.
\( 2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g) \)
The student uses the apparatus shown in Fig. 5.1.
Fig. 5.1
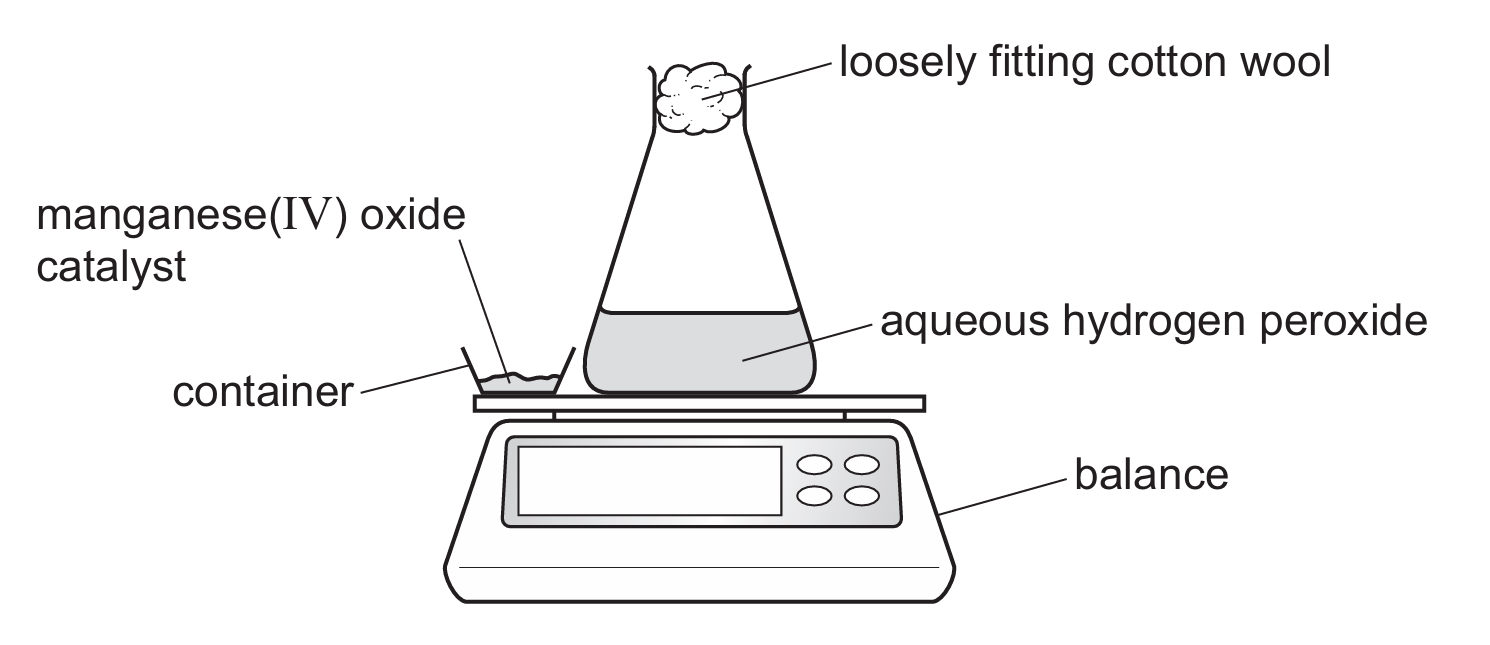
The student:
• adds the catalyst to the aqueous hydrogen peroxide
• replaces the container on the balance
• starts a stop-watch
• records the mass at regular time intervals.
(a) Table 5.1 shows the mass recorded at regular time intervals.
Table 5.1
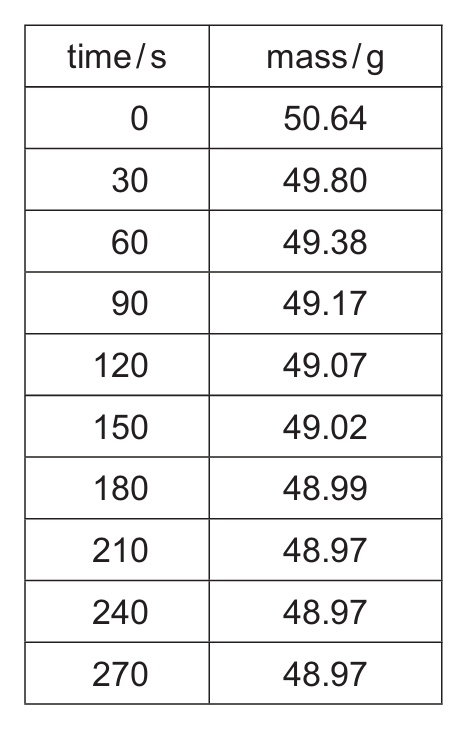
(i) Suggest why the mass decreases as time increases.
(ii) After a certain time the reaction stops. Explain why the reaction stops.
(iii) Suggest why it is not possible to use the results in Table 5.1 to determine the exact time when the reaction stops.
(b) Fig. 5.2 shows a graph of the mass against time.
Fig. 5.2
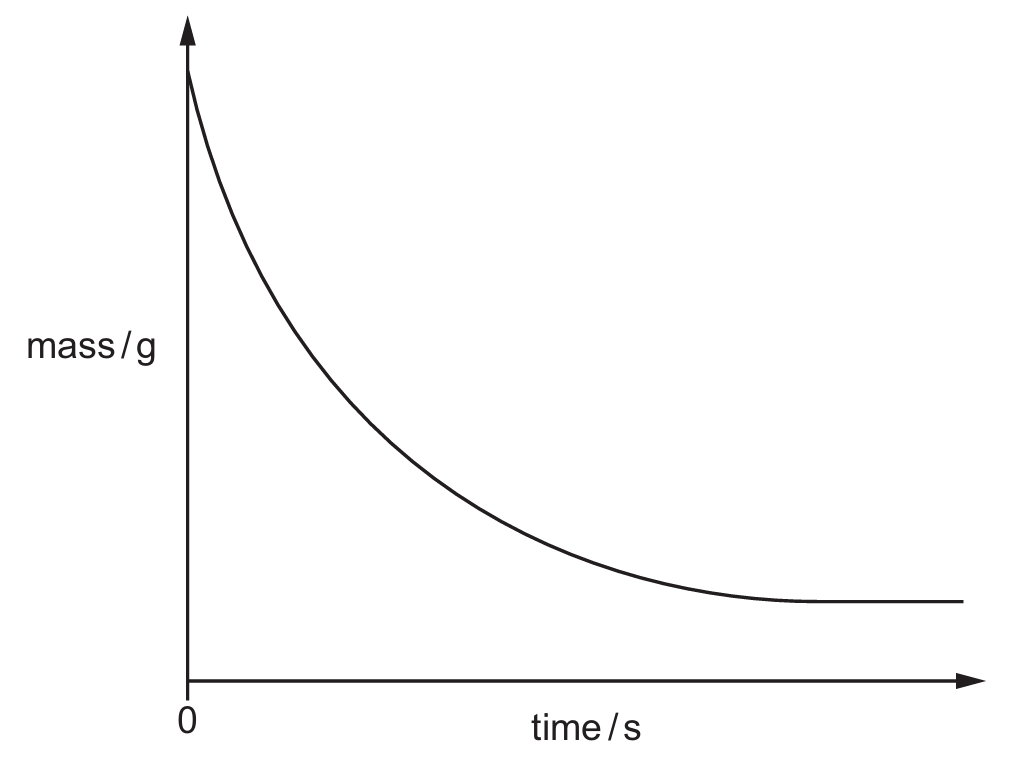
The experiment is repeated at a higher temperature. All other conditions remain the same.
(i) Explain, in terms of collision theory, why the rate of reaction is higher at a higher temperature.
(ii) On Fig. 5.2, sketch the line expected when the experiment is repeated at a higher temperature.
(c) Manganese(IV) oxide is the catalyst in this reaction.
(i) Explain the meaning of (IV) in manganese(IV) oxide.
(ii) State how the mass of the catalyst has changed, if at all, at the end of the experiment.
(d) Nitrogen monoxide gas, \( NO \), and oxygen gas, \( O_2 \), react to produce nitrogen dioxide gas, \( NO_2 \), at room temperature.
The reaction can reach equilibrium. The equation is shown.
\( 2NO(g) + O_2(g) \rightleftharpoons 2NO_2(g) \) \( \Delta H = -113 \, \text{kJ/mol} \)
NO(g) and \( O_2(g) \) are passed into a beaker as shown in Fig. 5.3.
Fig. 5.3
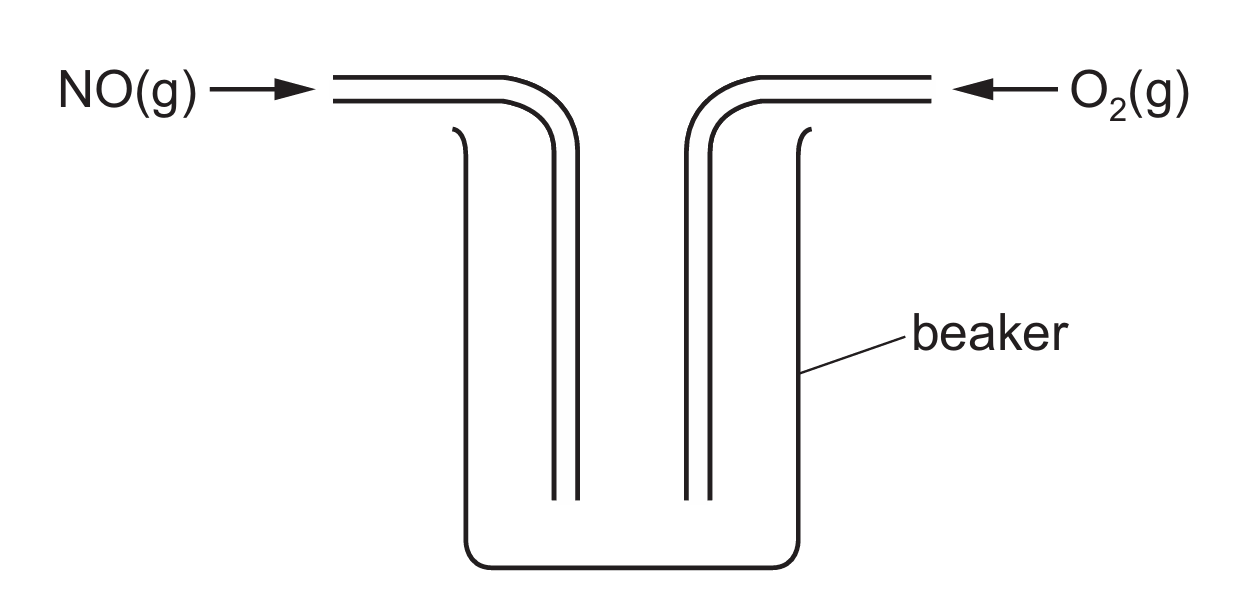
(i) Explain why the method shown in Fig. 5.3 will not allow the reaction to reach equilibrium.
(ii) The apparatus is changed and equilibrium is reached. The temperature of the equilibrium system is then increased and the position of equilibrium shifts to the left. Explain why the position of equilibrium shifts to the left.
(iii) The pressure of the equilibrium system is then increased. State the direction, if any, in which the position of equilibrium shifts. Explain your answer.
▶️ Answer/Explanation
(a)(i) Oxygen gas escapes from the flask.
The decomposition of hydrogen peroxide produces oxygen gas, which is allowed to escape through the loosely fitting cotton wool. As the gas escapes, the mass of the system decreases.
(a)(ii) The hydrogen peroxide is used up.
All the hydrogen peroxide has reacted or decomposed. Since the reaction requires hydrogen peroxide as a reactant, when it’s completely consumed, the reaction stops.
(a)(iii) Time intervals are too large.
The mass readings are taken at 30-second intervals, which may be too infrequent to pinpoint the exact moment when the reaction stops. The mass becomes constant between 210-270 seconds, but we can’t determine the exact time within this range.
(b)(i)
1. Kinetic energy of particles increases.
2. Frequency of collisions between particles increases.
3. More particles have energy greater than or equal to activation energy.
At higher temperatures, particles move faster and collide more frequently with greater energy, increasing the likelihood of successful collisions that lead to reaction.
(b)(ii)
1. Graph starts at same mass and has steeper gradient.
2. Levels off at the same mass but earlier.
The higher temperature causes a faster initial rate (steeper slope) but the reaction reaches completion sooner as reactants are used up more quickly.
(c)(i) Oxidation number of manganese is +4.
The Roman numeral (IV) indicates the oxidation state of manganese in this compound. Since oxygen typically has an oxidation state of -2, and the formula is MnO₂, the manganese must be +4 to balance the charges.
(c)(ii) No change.
Catalysts are not consumed in reactions. While manganese(IV) oxide participates in the reaction mechanism, it is regenerated and its mass remains unchanged at the end.
(d)(i) Not a closed system.
The beaker is open, allowing gases to escape. Equilibrium can only be established in a closed system where no reactants or products can enter or leave.
(d)(ii) Forward reaction is exothermic.
According to Le Chatelier’s principle, increasing temperature favors the endothermic direction. Since the forward reaction is exothermic (\(\Delta H\) negative), increasing temperature shifts equilibrium to the left (endothermic reverse reaction).
(d)(iii)
Direction: to the right.
Explanation: There are fewer gas molecules on the right (2 moles of NO₂) compared to the left (2 moles of NO + 1 mole of O₂ = 3 moles total). Increasing pressure shifts equilibrium to the side with fewer gas molecules.
A student adds excess large pieces of magnesium carbonate, MgCO3, to dilute hydrochloric acid, HCl, and measures the volume of carbon dioxide gas, CO2, given off.
(a) Add the missing state symbols to the chemical equation for the reaction.
MgCO3 ….. + 2HCl….. → MgCl2(aq) + H2O ….. + CO2 …..
(b) Complete the dot-and-cross diagram to show the electron arrangement of the ions in magnesium chloride.
The inner shells have been drawn.
Give the charges on the ions.

(c) Complete the dot-and-cross diagram to show the electron arrangement in a molecule of carbon dioxide.
Show outer shell electrons only.

(d) The graph shows how the volume of carbon dioxide gas changes with time.

(i) Describe how the graph shows that the rate of this reaction decreases as time increases.
(ii) Explain, in terms of particles, why the rate of this reaction decreases as time increases.
(iii) The student repeats the experiment using powdered MgCO3 instead of large pieces.
All other conditions stay the same.
On the grid, draw the line expected when powdered MgCO3 is used instead of large pieces.
(e) Determine the volume of CO2 gas given off when excess MgCO3 is added to 25.0cm3 of 0.400mol/dm3 HCl at room temperature and pressure.
MgCO3 + 2HCl → MgCl2 + H2O + CO2
Use the following steps.
- Calculate the number of moles of HCl in 25.0cm3 of 0.400mol/dm3 of acid.
- Determine the number of moles of CO2 gas given off.
- Calculate the volume of CO2 gas given off in cm3.
▶️ Answer/Explanation
(a) Ans: MgCO3(s) + 2HCl(aq) → MgCl2(aq) + H2O(l) + CO2(g)
Magnesium carbonate is a solid (s), hydrochloric acid is aqueous (aq), water is liquid (l), and carbon dioxide is a gas (g).
(b) Ans: Eight crosses in the second shell of Mg, 7 dots and 1 cross in the third shell of both Cl. Mg2+ and Cl− charges.
Magnesium loses 2 electrons (forming Mg2+), and each chlorine gains 1 electron (forming Cl−).
(c) Ans: C atom double bonded to 2 O atoms, with 4 non-bonding electrons on each O and no non-bonding electrons on C.
CO2 has a linear structure with two double bonds, satisfying the octet rule for all atoms.
(d)(i) Ans: The gradient of the line decreases over time.
The slope represents the reaction rate, and a decreasing slope indicates a slower rate.
(d)(ii) Ans: The concentration of HCl decreases, leading to fewer successful collisions.
As the reaction progresses, HCl is used up, reducing the frequency of effective collisions between particles.
(d)(iii) Ans: A steeper line starting at the origin, leveling off earlier but reaching the same final volume.
Powdered MgCO3 has a larger surface area, increasing the initial reaction rate.
(e) Ans: 120 cm3
Moles of HCl = 0.400 × 0.025 = 0.01 mol. Moles of CO2 = 0.01 / 2 = 0.005 mol. Volume = 0.005 × 24000 = 120 cm3.
