Question:
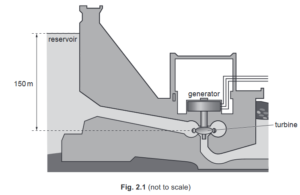
Fig. 2.1 shows water stored in a reservoir behind a hydroelectric dam.
(a) State the form of the energy stored in the water in the reservoir that is used to generate electricity.
Answer/Explanation
Ans: gravitational potential energy
(b) The turbine is 150 m below the level of the water in the reservoir.
Atmospheric pressure is 1.0 × 10 5 Pa. The density of water is 1000 kg / m3 .
(i) Calculate the total pressure in the water at the turbine.
pressure = …………………………………………………
Answer/Explanation
Ans: 1.6 × 10 6 Pa
(p =) hρ g (in any form) or 150 × 1000 × 10 or 1.5 × 10 6
1.5 × 10 6 or 1.0 × 10 5 + {150 × 1000 × 10} or 1.0 × 10 5 + 1.5 × 10 6
or 1.6 × 10N
(ii) The turbine has a cross-sectional area of 3.5 m2 .
Calculate the force exerted on the turbine by the water.
force = …………………………………………………
Answer/Explanation
Ans: 5.6 × 10 6 N
(F =) pA (in any form) or 1.6 × 10 6 × 3.5
(c) The water flows to the turbine through a pipe of constant cross-sectional area.
Explain why the kinetic energy of the water in the pipe remains constant as it flows through the pipe.
Answer/Explanation
Ans: speed (of water) remains constant
otherwise density would decrease or gaps would appear in the water
or volume / density does not change or liquids incompressible or water enters / leaves at constant rate or quantity of water
remains constant
Question
A manometer is being used to measure the pressure of the gas in a container, as shown in Fig. 6.1.
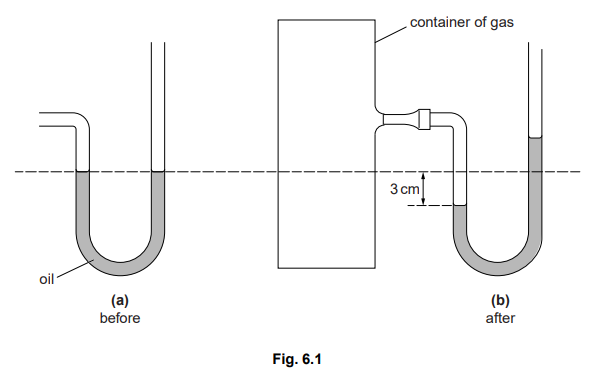
(a) The appearance of the oil in the manometer before connecting it to the container is
shown in Fig. 6.1(a).
Explain why the oil levels are the same in both limbs of the manometer.
…………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………….. [1]
(b) Fig. 6.1(b) shows the oil levels after connecting to the container.
By how much does the gas pressure in the container differ from atmospheric pressure?
Tick one box.
 [1]
[1]
(c) When the gas in the container is heated, the pressure rises.
(i) What happens to the oil level
1. in the left-hand limb, ………………………………………………………………………………
2. in the right-hand limb? …………………………………………………………………………..[1]
(ii) Explain, in terms of molecules, why the pressure of the gas rises when it is heated.
………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………….
……………………………………………………………………………………………………………… [3] [Total: 6]
Answer/Explanation
Ans: (a) same pressure
(b) 6 cm of oil greater
(c) (i) 1. falls / decreases / down both needed 2. rises / increases / up }both needed
(ii) they move faster / more energetically o.w.t.t.e. collisions more frequent/often or harder any 3 points collisions with walls/container/sideslarger force (on wall/container)} any 3 points
Question
(a) Explain
(i) how gas molecules exert a force on a solid surface,
(ii) the increase in pressure of a gas when its volume is decreased at constant temperature.
(b) A cylinder of volume \(5.0 × 10^3 cm^3\) contains air at a pressure of \(8.0 × 10^5\) Pa.
A leak develops so that air gradually escapes from the cylinder until the air in the cylinder is at atmospheric pressure. The pressure of the atmosphere is \(1.0 × 10^5\) Pa.
Calculate the volume of the escaped air, now at atmospheric pressure. Assume that the temperature stays constant.
volume = ……………………… \(cm^3\)
Answer/Explanation
Answer:
(a) (i) (Force exerted when) molecules hit wall / surface / solid (and rebound) Allow (force) due to momentum change in collision
(ii) Molecules/atoms/particles collide with / push against walls more (often)
(so) bigger force / push
NOT collide faster
(b) \(P_1 V_1=P_2 V_2\) OR PV = constant
\(8.0 \times 10^5 \times 5000=1 \times 10^5 \times V_2\)
\(V_2=40000 cm^3\)
Volume escaped =40000-5000=35000 \(cm^3\)
