Question
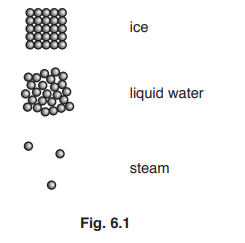
Water can exist as ice, liquid water and steam. Fig. 6.1 represents the arrangement of the molecules in the three forms of water.
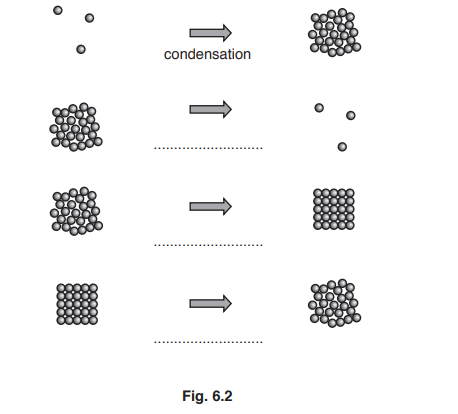
(a) Each diagram in Fig. 6.2 shows a change of state.
Add the correct label for each change. The first has been done for you.
(b) Some gas is heated at constant pressure.
Describe what happens to the molecules of gas as the temperature increases.
(c) Fig. 6.3 shows a metal bar.
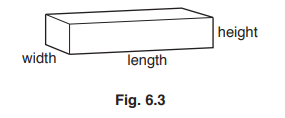
When the metal bar is heated, the bar expands.
Identify the dimensions that increase in size when the bar is heated.
Tick (√) all boxes that apply.
[1]
(d) State one use and one disadvantage of the expansion of materials when they are heated.
use …………………………………………………………………………………………………………………………..
disadvantage …………………………………………………………………………………………………………….
Answer/Explanation
Answer:
(a) evaporation / boiling
solidification/ freezing
melting
(b) faster movement / gain kinetic energy
larger separation of molecules owtte
(c) all boxes ticked
(d) bimetal strips
train rails buckling
Question
A small cylinder of compressed helium gas is used to inflate balloons for a celebration.
(a) (i) In the box below, sketch a diagram to represent the arrangement of helium molecules in
a balloon.
(ii) State and explain how the size of the attractive forces acting between the molecules of a
gas compares with the size of the attractive forces between the molecules of a solid.
(b) The helium in the cylinder has a volume of \(6.0 × 10^{–3} m^3\) (0.0060 \(m^3\)) and is at a pressure of
\(2.75 × 10^6\) Pa.
(i) The pressure of helium in each balloon is \(1.1 × 10^5\) Pa. The volume of helium in an inflated
balloon is \(3.0 × 10^{–3}\) (0.0030 \(m^3\)). The temperature of the helium does not change.
Calculate the number of balloons that were inflated.
number of balloons =
(ii) Later, the temperature increases and some of the balloons burst.
Suggest and explain why this happens.
Answer/Explanation
Answer:
(a) (i) diagram showing:
molecules widely spaced
molecules randomly positioned
(ii) (attractive) forces (much) smaller between gas molecules
gas molecules (much) farther apart
(b) (i) pV = constant OR \(p_1V_1 = p_2V_2\) OR \((V_2 =) p_1V_1/ p_2\)
OR \((V_2 =) 2.75 × 10^6 × 6 × 10^{–3}/ 1.1 × 10^5\)
= 0.15 \(m^3\)
(no. of balloons = \((0.15 – 6 × 10^{–3})/ 3 × 10^{–3} =) 48\)
(ii) pressure of air in balloon increases
molecules move faster OR hit balloon surface harder/ more often
OR larger force rips / breaks rubber OR balloon expands
