Question:
A thermocouple is a device that is used as a thermometer.
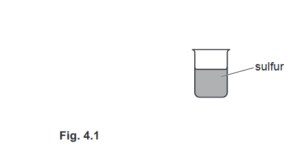
(a) Fig. 4.1 shows a beaker that contains molten sulfur at an initial temperature greater than 400 °C.
(i) On Fig. 4.1, sketch and label a diagram of a thermocouple that is used to determine the temperature of the sulfur as it cools to room temperature.
Answer/Explanation
Ans: two / three wires of at least two different metals
one junction in sulfur
the other junction in ice-water mixture / at room temperature and one of the wires must be from the first junction
labelled voltmeter / voltmeter symbol correctly connected
(ii) Describe briefly how the temperature of the sulfur in the beaker is deduced.
Answer/Explanation
Ans: measure e.m.f.
how to find temperature from e.m.f. (e.g. use calibration graph or calculation or table)
(b) State one advantage of using a thermocouple to measure temperature rather than using a liquid-in-glass thermometer.
Answer/Explanation
Ans: measures high temperatures / wires do not melt / rapid response / robust / small heat capacity / electrical output / (can be) remote from observer / direct input to computer
Question
Fig. 4.1 shows a typical laboratory liquid-in-glass thermometer.
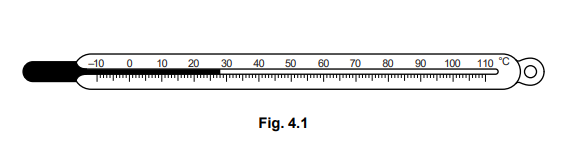
(a) Name a liquid that is likely to be used in this thermometer.
…………………………………………………………………………………………………………………….. [1]
(b) What occupies the space in the tube, between the end of the liquid thread and the end of the tube?
…………………………………………………………………………………………………………………….. [1]
(c) On Fig. 4.1, clearly indicate and label
1. the ice point,
2. the steam point. [2]
(d) The thermometer is moved into a hotter place.
(i) State what happens to the position of the end of the liquid thread.
………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………….
(ii) Explain why this happens.
………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………….
[2] [Total: 6]
Answer/Explanation
Ans: (a) mercury/Hg OR alcohol OR named alcohol e.g. ethanol
(b) vacuum OR nothing OR empty OR vapour
(c) ice point indicated and labelled at 0°C steam point indicated and labelled at 100°C
(d) (i) moves to the right (or equivalent e.g. goes higher/up/rises)
(ii) liquid expands NOT thermometer/particles expands
Question
Fig. 5.1 shows the structure of a liquid-in-glass thermometer.
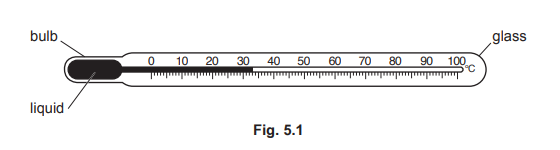
The bulb of the thermometer is placed into a beaker of warm water. As the liquid expands, it moves along the tube.
(a) Explain, in terms of molecules, why a liquid expands when heated.
(b) Explain, in terms of molecules, why a liquid expands more than a solid when heated.
(c) A second thermometer has a larger bulb that contains more of the same liquid than the thermometer shown in Fig. 5.1. It has a different scale. In every other way, it is identical.
(i) Explain how the sensitivity of the second thermometer compares with the sensitivity of the thermometer in Fig. 5.1.
(ii) Explain how the range of the second thermometer compares with the range of the thermometer in Fig. 5.1.
(d) (i) State one everyday problem that is a result of thermal expansion.
(ii) Suggest and explain one way of solving this problem.
Answer/Explanation
Answer:
(a) molecules / they speed up or gain kinetic energy
molecules move further apart or push others away
(b) forces between liquid molecules weak(er than in solids)
less energy / work done to separate molecules or greater separation for same work done / same increase in energy
(c)(i) greater sensitivity
volume increase (of liquid in second thermometer) is greater
or liquid moves a greater distance (for the same temperature increase)
(c)(ii) smaller range and either of:
• smaller temperature increase for liquid / meniscus to reach end of tube
• expands more / greater sensitivity and tube of same length
(d)(i) statement of problem (e.g. bridges buckle (in hot weather))
(d)(ii) suggested solution to problem stated in (e.g. allow gaps at the ends of the bridge)
more detail (e.g. as the bridge expands the gaps close)
