Question:
A teacher fills a copper can with solid wax and heats the can. She measures the temperature of the wax every minute. She continues heating once the wax has melted and stops heating when
the wax is boiling.
(a) (i) State the term used for the process that transfers thermal energy through the copper.
Answer/Explanation
Ans: conduction
(ii) Fig. 6.1 shows how the temperature of the wax changes as it is heated.
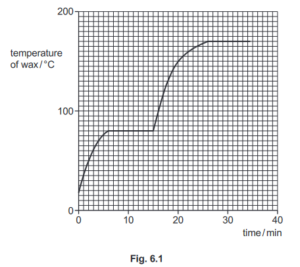
Using the graph in Fig. 6.1, determine:
1. the melting point of the wax ……………………………………………… °C
Answer/Explanation
Ans: 80 (°C)
2. the boiling point of the wax ……………………………………………… °C
Answer/Explanation
Ans: 170 (°C)
3. the time at which the wax starts to boil. ……………………………………………. min
Answer/Explanation
Ans: 26 (minutes)
(b) Describe the molecular structure of the wax in terms of the arrangement, separation and
motion of its molecules when it is a solid and when it is a gas.
solid wax …………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………
wax as a gas ……………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………………..
Answer/Explanation
Ans: (solid:) particles/molecules any three from:
(are) fixed in place/position/arrangement
regular spacing / pattern / arrangement
vibrating
close together
(gas:) particles/molecules any three from:
(are) moving randomly
at high speed
colliding (with each other/walls)
randomly arranged/no pattern
(relatively) far apart
Question
A liquid-in-glass thermometer is placed in some ice made from pure water. The ice is heated. It changes to water and then to steam.
The graph in Fig. 6.1 shows how the temperature varies with time. The values of temperature are missing from the y-axis.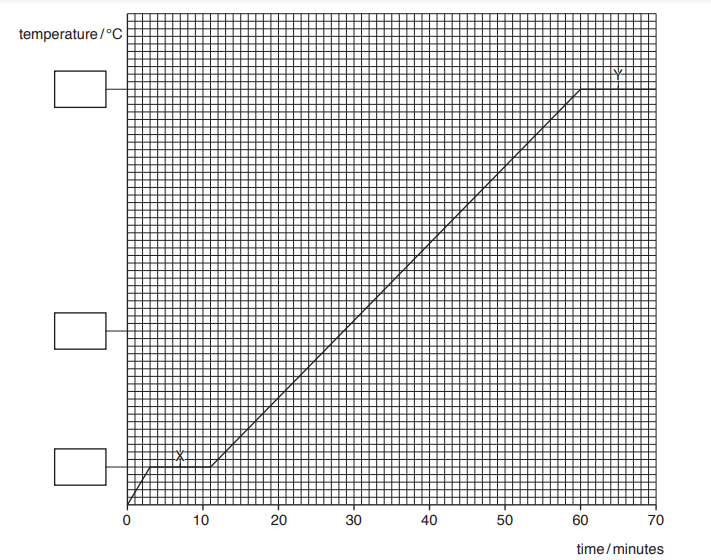
(a) On Fig. 6.1, suggest a value for the temperature at each of the three points marked on the y-axis.
Write a value in each of the boxes.
(b) In both section X and section Y the line on the graph is horizontal.
For each section, state the name for the process taking place and explain what is happening to the molecules.
i. section X
name
explanation
ii. section Y
name
explanation
Answer/Explanation
Answer:
(a) 0 AND 100 correctly labelled
36
(b) (i) Melting
Any one of:
molecules gain energy
molecule (begin to) break (some) bonds
arrangement becomes irregular or arrangement changes
(ii) boiling
Any one of:
molecules break (all) bonds
molecules move (more) freely
molecules become widely separated or far apart
Question
(a) On a hot day, sweat forms on the surface of a person’s body and the sweat evaporates.
Explain, in terms of the behaviour of molecules,
(i) the process of evaporation,
(ii) how this process helps the body to cool down.
(b) The temperature of a person of mass 60 kg falls from 37.2 °C to 36.7 °C.
(i) Calculate the thermal energy lost from the body. The average specific heat capacity of the body is 4000 J / (kg °C).
thermal energy lost = ………………………………………..
(ii)The cooling of the body was entirely due to the evaporation of sweat.
Calculate the mass of sweat which evaporated. The specific latent heat of vaporisation of sweat is 2.4 × \(10^5\) J / kg.
mass =……………………………………
Answer/Explanation
Answer:
(a) (i) and (ii) marked together to maximum of 3 marks
(i) molecules escape/leave the liquid/form gas or vapour
(ii) evaporation OR heat/(thermal) energy needed for evaporation leaves sweat cooler fast(er) molecules/high(er) energy molecules escape
OR slow(er) molecules left behind heat flows from body to warm the sweat (so body cools)
(b) (i) (Q =) mc∆θ OR mcT OR 60 × 4000 × 0.50 1.2 × \(10^5\) J / 120 kJ
(ii) Q = mL in any form OR (m =) Q/L OR either with numbers (m = 1.2 × 105 / 2.4 × \(10^6\) =) 0.05 kg e.c.f from (b)(i)
Question
Fig. 6.1 shows workers pouring liquid metal.
(a) The metal changes from hot liquid to cool solid.
Describe what happens to the arrangement, separation and motion of the atoms as the metal changes from hot liquid to cool solid.
(b) The workers cool their tools in water. They spill some water onto the floor but later the floor is dry.
Explain what happens to the water. State the name of the process.
explanation ………..
process ……….
Answer/Explanation
Answer:
(a) more regular/uniform arrangement/fixed position owtte
separation between atoms decreases/move closer/tightly packed
slower moving atoms/atoms vibrate (more slowly)
(b) (water) molecules gain energy (from surroundings)
molecules escape from a liquid (surface)
evaporation
