Question
A nucleus of americium-241 has the nuclide notation shown.
\(^{241}_{95}\) Am
(a) (i) Determine the number of neutrons in a nucleus of americium-241.
number of neutrons =…………………………………………………………………………………………….
(ii) Determine the charge on a nucleus of americium-241.
charge =………………………………………………………………………………………………….
(b) Americium-241 decays by emitting α-particles.
Put a tick in the box next to each correct statement.

(c) Americium-241 has a half-life of 432 years.A sample contains 16 mg of americium-241.Calculate the time it takes until only 4.0 mg of americium-241 are left in the sample.
time = ………………………………………. years
Answer/Explanation
Answer:
(a)
(i) 146
(ii) positive
95
(b) tick in 3rd box
tick in 4th box
(c) idea of 2 half-lives
(432 × 2) = 864 (years)
Question
Three types of ionising radiation are alpha, beta and gamma.
(a) Draw one straight line from each type of radiation to a property of that radiation.
(b) Polonium-210 has the nuclide notation \(^{210}_{84}\)Po.
For one neutral atom of polonium-210,
(i) determine the number of protons,………………………………………………………
(ii) determine the number of neutrons …………………………………………………….
(c) Fig. 12.1 shows a decay curve for polonium-210.
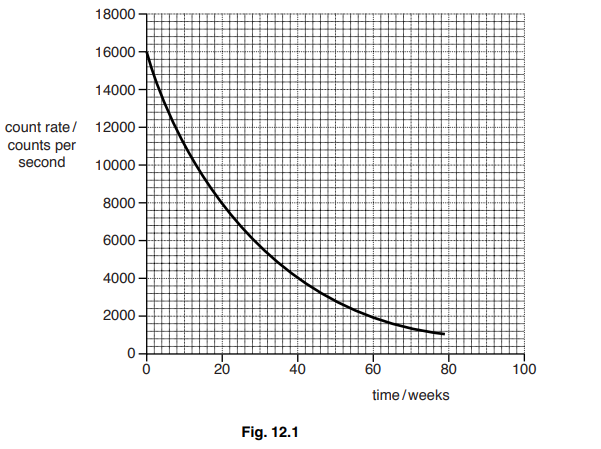
Use the graph to determine the half-life of polonium-210.
half-life =……………………………………………………………………………………………………………… weeks
Answer/Explanation
Answer:
(a) line from alpha to stopped by paper
line from beta to negative charge
line from gamma to e.m. radiation
(b) (i) 84
(ii) 126
(c) evidence of line from 8000 or idea of halving e.g. 8000 and 4000
20 ± 1.0 (weeks)
