Question
The table below gives details about some radioactive substances.
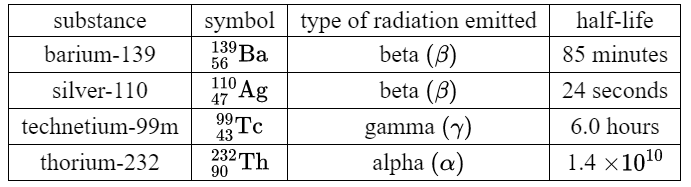
(a) Which of these substances has the greatest number of particles in the nucleus of its atoms?
……………………………………………………………………………………………………………………. [1]
(b) Which of these substances has the least number of electrons in the orbits of a neutral atom?
……………………………………………………………………………………………………………………. [1]
(c) Which of these substances are emitting particles?
…………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………. [2]
(d) Samples of each of these substances are decaying. Each sample starts with the same
number of atoms.
Which sample decays the most in one hour?
……………………………………………………………………………………………………………………. [1]
(e) In the investigation of a blood circulation problem, a patient is given an injection containing one of these substances. The radiation needs to be detectable from outside the body.
Which of the substances might be suitable for this use?
……………………………………………………………………………………………………………………. [1] [Total: 6]
Answer/Explanation
Ans: (a) thorium OR Th OR 232 OR 90
(b) technetium OR Tc OR 99(m) OR 43
(c) barium OR Ba OR 139 OR 56 silver OR Ag OR 110 OR 47 thorium OR Th OR 232 OR 90 } any 2 NOTE: technetium + anything scores 1 mark, “all of them” scores 1 mark
(d) silver OR Ag OR 110 OR 47
(e) technetium OR Tc OR 99(m) OR 43 OR gamma
NOT any extras
Question
(a) State the proton number, nucleon number and the value of the charge on an α-particle.
proton number …………………………………………………………………………………………………………..
nucleon number …………………………………………………………………………………………………………
charge ………………………………………………………………………………………………………………………
(b) A nucleus of strontium-90 consists of 38 protons and 52 neutrons. Strontium-90 is radioactive
and decays by β-emission to an isotope of yttrium. The symbol for strontium is Sr and the
symbol for yttrium is Y. Write down the nuclide equation of this decay.
(c) The half-life of radon-220 is 56 s. A sample of radon-220 is in a container. After 112 s the mass
of radon-220 is 9.2 mg.
Calculate the mass of the original sample.
mass = ……………………………………………..
Answer/Explanation
Answer:
(a)
2
4
+2
(b) 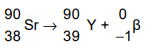
nucleon numbers 90 on both sides of equation
Sr and proton number 38 on left AND Y and proton number 39 on right 
(c) (original mass = 4 / 9.2 =) 37 mg
2 half-lives
