Question
Air in a sealed syringe is slowly compressed by moving the piston. The temperature of the air
stays the same.
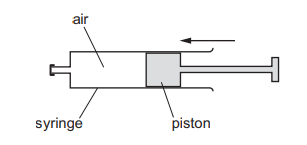
Which statement about the air is correct?
A The pressure of the air decreases because its molecules now travel more slowly.
B The pressure of the air decreases because the area of the syringe walls is now smaller.
C The pressure of the air increases because its molecules now hit the syringe walls more frequently.
D The pressure of the air increases because its molecules now travel more quickly.
Answer/Explanation
Ans: C
Question
The diagram shows a mercury manometer used to measure the pressure of gas in a container.
Atmospheric pressure is 76 cm of mercury.
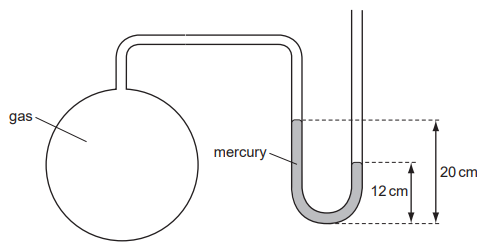
What is the pressure of the gas?
A 56 cm of mercury
B 68 cm of mercury
C 84 cm of mercury
D 96 cm of mercury
Answer/Explanation
Ans: B
Question
A gas storage tank has a fixed volume. The graph shows how the temperature of the gas in the
tank varies with time.
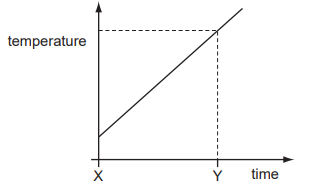
At time Y, the gas molecules are
A. closer together than at time X.
B. hitting the sides of the tank harder than at time X.
C. larger in size than at time X.
D. moving more slowly than at time X.
Answer/Explanation
Ans: B
Question
A student places his thumb firmly on the outlet of a bicycle pump, to stop the air coming out.
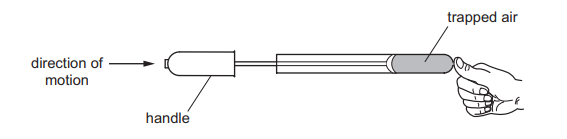
What happens to the pressure and what happens to the volume of the trapped air as the pump handle is pushed in?
| pressure | volume |
A | decreases | decreases |
B | decreases | remains the same |
C | increases | decreases |
D | increases | remains the same |
Answer/Explanation
Ans: C
Question
The pressure of a fixed mass of gas in a cylinder is measured. The temperature of the gas in the
cylinder is then slowly increased. The volume of the cylinder does not change.
Which graph shows the pressure of the gas during this process?
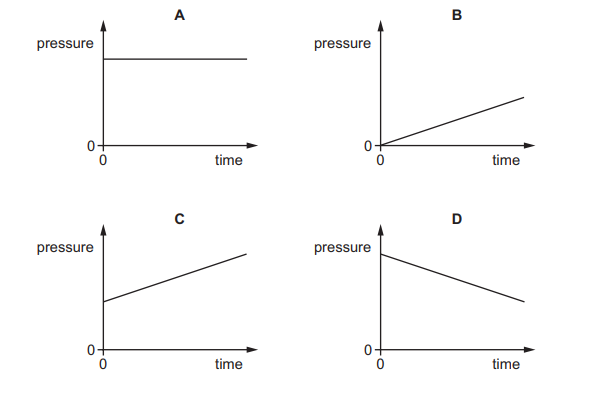
Answer/Explanation
Ans: C
