Question
The diagram shows the apparatus needed for an experiment to determine the specific heat capacity of the material from which an object is made.
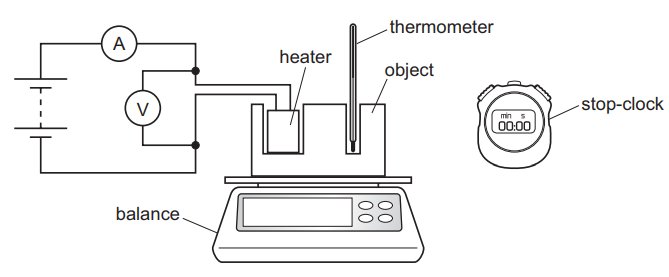
Which piece of apparatus could be omitted if the purpose of the experiment is to determine the thermal capacity of the object?
- ammeter
- balance
- stop-clock
- thermometer
Answer/Explanation
Ans:
B
Question
The diagram shows the apparatus needed for an experiment to determine the specific heat capacity of the material from which an object is made.

Which piece of apparatus could be omitted if the purpose of the experiment is to determine the thermal capacity of the object?
- ammeter
- balance
- stop-clock
- thermometer
Answer/Explanation
Ans:
B
Question
One end of a rod of copper is placed in hot water. Thermal energy travels along the rod to make the other end warmer.
What is the behaviour of the copper at an atomic level that accounts for most of the transfer of thermal energy from one end to the other?
A Atoms at the hot end gain kinetic energy and move towards the other end.
B Atoms at the hot end expand, colliding with other atoms and transferring energy.
C Free electrons at the hot end gain energy and move towards the other end, colliding with atoms along the rod.
D Free electrons at the hot end gain energy from the hot water and move directly to the other end.
Answer/Explanation
Ans:C
Question
One end of a rod of copper is placed in hot water. Thermal energy travels along the rod to make the other end warmer.
What is the behaviour of the copper at an atomic level that accounts for most of the transfer of thermal energy from one end to the other?
A Atoms at the hot end gain kinetic energy and move towards the other end.
B Atoms at the hot end expand, colliding with other atoms and transferring energy.
C Free electrons at the hot end gain energy and move towards the other end, colliding with atoms along the rod.
D Free electrons at the hot end gain energy from the hot water and move directly to the other end.
Answer/Explanation
Ans:C
Question
The graph shows how the internal energy of 1.0 kg of a metal changes with temperature.
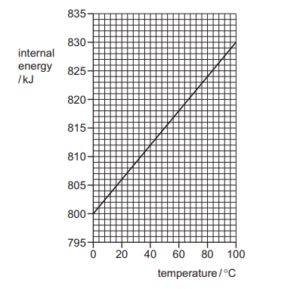
What is the increase in the internal energy of a block of the same metal of mass 0.25 kg when its temperature rises from 40 0C to 50 0C?
A 30 J
B 300 J
C 750 J
D 1200 J
Answer/Explanation
Ans: C
