Question
Both boiling and evaporation involve a change of state from liquid to gas.
Which row gives the correct difference between boiling and evaporation?
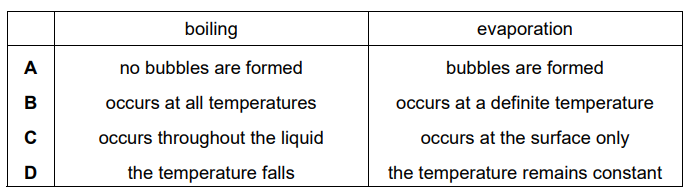
Answer/Explanation
Ans:
C
Question
Which change in the design of a liquid-in-glass thermometer makes it more sensitive?
A a larger liquid reservoir
B a longer tube
C a smaller liquid reservoir
D a wider tube
Answer/Explanation
Ans:A
Question
A liquid turns into a gas. This occurs only at one particular temperature, and the change happens throughout the liquid.
What is this process called?
A boiling
B condensation
C evaporation
D fusion
Answer/Explanation
Ans:A
Question
Two freezers X and Y are identical except that one has a door opening at the front and the other has a door opening at the top.
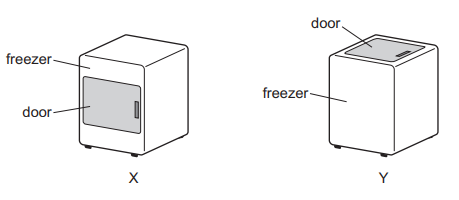
Both doors are the same size and are opened for the same amount of time.
Which freezer gains the least amount of thermal energy in this time and why?
|
freezer gaining the least thermal energy |
reason |
|
|
A |
X |
cold air falls |
|
B |
X |
warm air falls |
|
C |
Y |
cold air falls |
|
D |
Y |
warm air falls |
Answer/Explanation
Ans: CQuestion
100 g of water at 25°C is poured into an insulating cup. 50 g of ice at 0 °C is added to the water.
The water is stirred until the temperature of the water has fallen to 0 °C.
18 g of ice remains unmelted.
The specific heat capacity of water is 4.2 J / g °C.
Which value does this experiment give for the specific latent heat of fusion of ice?
A 210 J / g
B 330 J / g
C 580 J / g
D 770 J / g
Answer/Explanation
Ans: B
Question
Which statement about the evaporation of a liquid is correct?
A The least energetic molecules escape from the surface and the temperature of the liquid decreases.
B The least energetic molecules escape from the surface and the temperature of the liquid increases.
C The most energetic molecules escape from the surface and the temperature of the liquid decreases.
D The most energetic molecules escape from the surface and the temperature of the liquid increases.
Answer/Explanation
Ans:C
Question
A student sets up four cans. Each can contains the same mass of water at 90 0C.
The cans are identical except for the outside surfaces.
Which can will cool down the fastest?
A dull, black surface
B dull, white surface
C shiny, black surface
D shiny, white surface
Answer/Explanation
Ans: A
Question
Liquid evaporates from a beaker.
What happens to the temperature of the remaining liquid and how does this temperature change
affect the rate of evaporation?
temperature | rate of evaporation | |
A | decreases | decreases |
B | decreases | increases |
C | increases | decreases |
D | increases | increases |
Answer/Explanation
Ans: A
Question
During evaporation, molecules escape rapidly from the surface of a liquid.
What happens to the average energy of the molecules of the remaining liquid and what happens
to the temperature of the remaining liquid?
| average energy of remaining molecules | temperature of remaining liquid |
A | decreases | decreases |
B | decreases | increases |
C | stays the same | decreases |
D | stays the same | increases |
Answer/Explanation
Ans: A
Question
Which statement about evaporation is correct?
A Evaporation causes the temperature of the remaining liquid to decrease.
B Evaporation does not occur from a cold liquid near its freezing point.
C Evaporation does not occur from a dense liquid, such as mercury.
D Evaporation occurs from all parts of a liquid.
Answer/Explanation
Ans: A
