Question
When a radioactive isotope is set up close to a counter, a count rate of 38 000 counts / s is obtained. The table shows the count rate from the isotope over a three-year period.
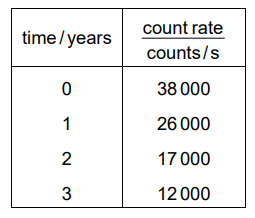
What is the half-life of the isotope?
- less than 1 year
- more than 1 year but less than 2 years
- more than 2 years but less than 3 years
- more than 3 years
Answer/Explanation
Ans:
B
Question
A laboratory worker measures the count rate from a radioactive source. He records his results in a table.
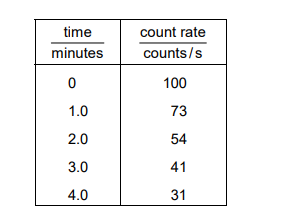
The average background radiation in the laboratory is 8 counts per second.
What is the half-life of the source?
A 1.5 minutes
B 2.0 minutes
C 3.0 minutes
D 4.0 minutes
Answer/Explanation
Ans: B
Question
Solid caesium-137 decays by the emission of a β-particle to form solid barium-137, which emits a γ-ray.
The barium-137 undergoes no further decay. The half-life of caesium-137 is 33 years.
A block of pure caesium-137 has a mass of 2.0 μg.
The diagram shows a radiation detector a distance of 5 cm from the block. The detector registers
a count rate of 2000 counts / second.
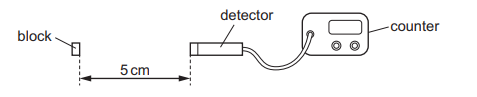
Which statement is not correct?
A After 33 years, the mass of the block is 1.0 μg.
B After 66 years, the sample contains 1.5 μg of barium.
C With 5 cm of lead between the block and the detector, the count rate is just above background level.
D With 2 mm of aluminium between the block and the detector, the count rate is reduced significantly.
Answer/Explanation
Ans: A
Question
A radioactive substance emits radiation at a rate of 600 emissions per second. Four hours later, it
emits radiation at a rate of 300 emissions per second.
What is the half-life of the substance and what is the rate of emission after a further four hours?
| half-life / hours | rate of emission after a further four hours / emissions per second |
A | 2 | 0 |
B | 2 | 150 |
C | 4 | 0 |
D | 4 | 150 |
Answer/Explanation
Ans: D
Question
The graph shows how the count rate registered by a counter near to a sample of a radioactive
isotope changes over a period of a few days. The background count rate is 5 counts per minute.
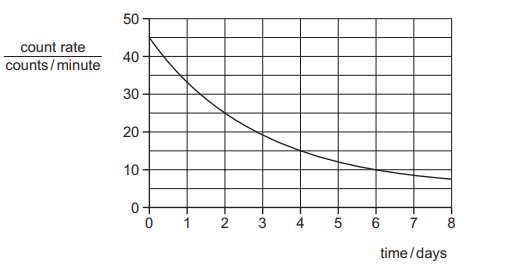
What is the half-life of the isotope?
A 2.0 days
B 2.5 days
C 3.0 days
D 4.0 days
Answer/Explanation
Ans: A
