Question
Which row describes the process of melting?
| initial state | final state | change in temperature? |
A | liquid | gas | yes |
B | liquid | solid | no |
C | solid | gas | yes |
D | solid | liquid | no |
Answer/Explanation
Ans: D
Question
The diagram shows the changes of state P, Q, R and S that occur in solids, liquids and gases
when they gain or lose thermal energy.
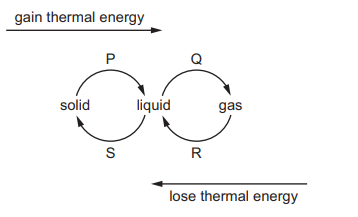
What is the name of change R?
A condensation
B solidification
C boiling
D melting
Answer/Explanation
Ans: A
AQuestion
A solid is heated from room temperature.
The graph shows how its temperature changes with time as it is heated constantly.
At which time has it just become completely liquid?
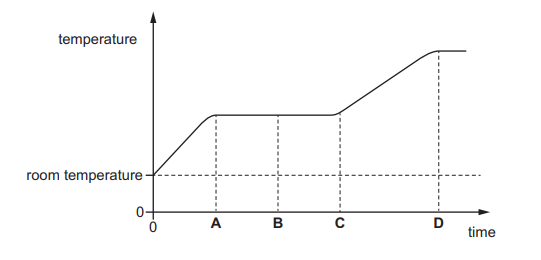
Answer/Explanation
Ans: C
Question
One ice cube is placed on a metal block. An identical ice cube is placed on a plastic block. The blocks are left next to each other on a table in a laboratory.
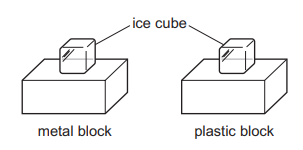
Which ice cube melts first and why?
The ice cube on the plastic block melts first because plastic is a good insulator of thermal energy.
The ice cube on the plastic block melts first because plastic is a good conductor of thermal energy.
The ice cube on the metal block melts first because metal is a good conductor of thermal energy.
The ice cube on the metal block melts first because metal is a good insulator of thermal energy.
Answer/Explanation
Ans: C
Question
In an experiment, a thermometer is placed in a test-tube of hot liquid. The temperature reading of the liquid is recorded every half minute. The table shows the results.
time / minutes | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 |
temperature / °C | 73 | 65 | 59 | 55 | 55 | 55 | 51 | 48 | 45 | 42 | 40 | 38 | 36 | 35 | 34 | 33 |
What is the melting point of the substance?
A 0 °C
B 33 °C
C 55 °C
D 73 °C
Answer/Explanation
Ans: C
Question
In an experiment, smoke particles are suspended in air and viewed through a microscope.
The smoke particles move about with short random movements. Which of the following statements is correct?
Air particles have large masses compared to smoke particles and they move in one direction only.
Air particles have large masses compared to smoke particles and they move in random directions.
Air particles move at high speeds compared to smoke particles and they move in one direction only.
Air particles move at high speeds compared to smoke particles and they move in random directions.
A beaker of liquid is heated slowly so that the liquid evaporates.
During evaporation, from where do the more energetic molecules leave the liquid?
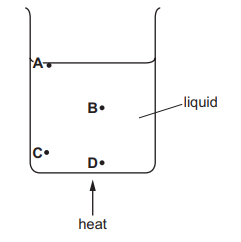
Answer/Explanation
Ans: A
Question
When a liquid evaporates, some molecules escape. The temperature of the remaining liquid changes.
What is the effect on the temperature and from where do the molecules escape?
| temperature of liquid | molecules escape from |
A | decreases | everywhere within the liquid |
B | decreases | the surface only |
C | increases | everywhere within the liquid |
D | increases | the surface only |
Answer/Explanation
Ans: B
