Acid–base titrations- CIE iGCSE Chemistry Notes - New Syllabus
Acid–base titrations for iGCSE Chemistry Notes
Core Syllabus
- Describe an acid–base titration to include the use of a:
(a) burette
(b) volumetric pipette
(c) suitable indicator - Describe how to identify the end-point of a titration using an indicator
Acid–base titration
Acid–base titration
- An acid–base titration is an experiment used to find the exact volume of one solution required to neutralise another
- It is a quantitative method, allowing the concentration of an unknown acid or alkali to be calculated
- The experiment requires accurate measuring apparatus and a suitable indicator to show the end-point
(a) Burette
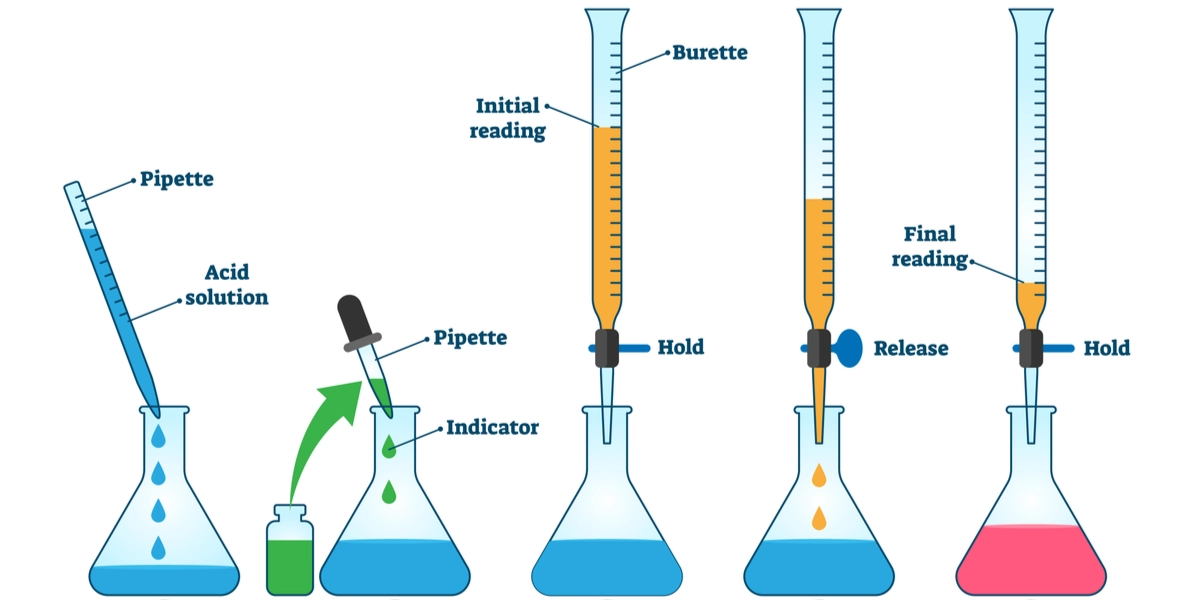
- A burette is a long graduated glass tube with a tap at the bottom
- It is clamped vertically to a stand, usually filled with the solution of known concentration (the titrant)
- The solution is run into a conical flask containing the other reactant until neutralisation occurs
- The burette allows precise control of liquid flow and accurate measurement to 0.1 cm³
- Common setup: acid in the burette and alkali in the flask, but the arrangement can be reversed
(b) Volumetric pipette
- A volumetric pipette delivers one fixed, accurate volume of solution (commonly 25.0 cm³)
- It is used to measure the solution of unknown concentration that will be placed in the conical flask
- A pipette filler is always used to draw liquid into the pipette safely
- The fixed volume ensures consistency and precision in each titration
(c) Suitable indicator
- An indicator is added to the solution in the conical flask to show when neutralisation is reached
- Only a few drops of indicator are required
- Common indicators:
- Phenolphthalein: colourless in acid, pink in alkali
- Methyl orange: red in acid, yellow in alkali, orange at end-point
- Choice of indicator depends on the type of acid and alkali:
- Strong acid + strong base: phenolphthalein or methyl orange can both be used
- Strong acid + weak base: methyl orange is more suitable
- Weak acid + strong base: phenolphthalein is more suitable
Step-by-step outline of titration
- Rinse and fill the burette with the standard solution (known concentration)
- Use a volumetric pipette to transfer a fixed volume of the solution of unknown concentration into a conical flask
- Add a few drops of the chosen indicator to the flask
- Run the solution from the burette slowly into the flask while swirling continuously
- As the end-point approaches, add drop by drop until the indicator changes colour
- Record the burette readings accurately to determine the titre (volume delivered)
Example
Why is a burette more suitable than a measuring cylinder for use in titrations?
▶️Answer/Explanation
A burette allows liquid to be added drop by drop and can measure volumes accurately to 0.1 cm³.
A measuring cylinder is much less precise and cannot deliver small controlled volumes, so it is unsuitable for titrations.
Example
Why is a pipette filler used when filling a volumetric pipette?
▶️Answer/Explanation
A pipette filler is used for safety reasons.
It avoids sucking solutions by mouth, which could be dangerous or toxic if swallowed or inhaled.
Example
Which indicator is most suitable for a titration between a strong acid and a weak base? Explain.
▶️Answer/Explanation
Methyl orange is suitable. In a strong acid–weak base titration, the pH at the end-point is below 7.
Methyl orange changes colour in the acidic range (red → orange → yellow), so it clearly shows the endpoint. Phenolphthalein would not give a sharp colour change in this case.
Example
During a titration, a student obtains titres of 23.6 cm³, 23.4 cm³ and 24.1 cm³. Which readings are concordant, and what volume should be used in calculations?
▶️Answer/Explanation
Concordant titres are those within 0.1 cm³ of each other.
Here, 23.6 cm³ and 23.4 cm³ are concordant.
The mean titre = \( \dfrac{23.6 + 23.4}{2} = 23.5 \,\text{cm}^3 \).
This value is used in calculations.
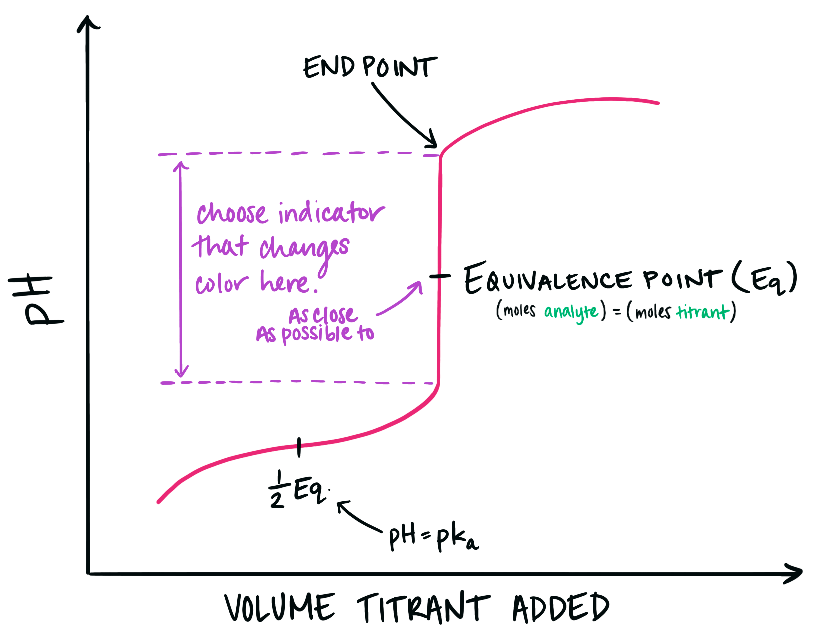
Identification of the end-point of a titration
Identification of the end-point of a titration
- The end-point of a titration is the exact stage at which the acid and base have completely reacted (neutralisation)
- It is detected using a suitable indicator that shows a sharp colour change when neutralisation is reached
- The colour change must be clear and easy to see

Using indicators
- A few drops of indicator are added to the solution in the conical flask at the start of the titration
- The burette solution is run in slowly while swirling the flask
- As the end-point approaches, the solution is added drop by drop
- The moment the indicator changes colour permanently, neutralisation is complete
- The burette reading at this point is recorded as the titre volume
Examples of colour changes
- Phenolphthalein: pink in alkali, colourless in acid. At the end-point, the solution changes from pink to colourless when the alkali has been neutralised
- Methyl orange: red in acid, yellow in alkali. At the end-point, the solution is orange as the transition occurs between red and yellow
Notes on reliability
- The end-point is more accurate if the titration is repeated several times until concordant results are obtained (titres within 0.1 cm³)
- Using the correct indicator for the strength of acid and base is essential for a sharp colour change
- If no suitable indicator exists (e.g., weak acid vs weak base), a pH probe or meter must be used instead of an indicator
Example
During an acid–base titration between hydrochloric acid and sodium hydroxide, how can the end-point of the reaction be identified?
▶️Answer/Explanation
Explanation:
- The end-point of a titration is the point where the acid and base have exactly neutralised each other.
- This is detected using a suitable indicator, which changes colour at or very close to the equivalence point.
Example with Indicator:
- If phenolphthalein is used: the solution in the flask is pink (alkaline) at the start. At the end-point, the last drop of acid causes the colour to change from pink to colourless.
- If methyl orange is used: the solution is yellow (alkaline) at the start. At the end-point, the last drop of acid turns the solution orange.
Final Answer: The end-point of a titration is identified by the sudden colour change of the indicator, showing that neutralisation has been achieved.