Air quality and climate- CIE iGCSE Chemistry Notes - New Syllabus
Air quality and climate for iGCSE Chemistry Notes
Core Syllabus
- State the composition of clean, dry air as approximately 78% nitrogen, N2, 21% oxygen, O2 and the remainder as a mixture of noble gases and carbon dioxide, CO2
- State the source of each of these air pollutants, limited to:
(a) carbon dioxide from the complete combustion of carbon-containing fuels
(b) carbon monoxide and particulates from the incomplete combustion of carbon-containing fuels
(c) methane from the decomposition of vegetation and waste gases from digestion in animals
(d) oxides of nitrogen from car engines
(e) sulfur dioxide from the combustion of fossil fuels which contain sulfur compounds - State the adverse effect of these air pollutants, limited to:
(a) carbon dioxide: higher levels of carbon dioxide leading to increased global warming, which leads to climate change
(b) carbon monoxide: toxic gas
(c) particulates: increased risk of respiratory problems and cancer
(d) methane: higher levels of methane leading to increased global warming, which leads to climate change
(e) oxides of nitrogen: acid rain, photochemical smog and respiratory problems
(f) sulfur dioxide: acid rain - State and explain strategies to reduce the effects of these environmental issues, limited to:
(a) climate change: planting trees, reduction in livestock farming, decreasing use of fossil fuels, increasing use of hydrogen and renewable energy, e.g. wind, solar
(b) acid rain: use of catalytic converters in vehicles, reducing emissions of sulfur dioxide by using low-sulfur fuels and flue gas desulfurisation with calcium oxide - Describe photosynthesis as the reaction between carbon dioxide and water to produce glucose and oxygen in the presence of chlorophyll and using energy from light
State the word equation for photosynthesis, carbon dioxide + water → glucose + oxygen
Supplement Syllabus
- Describe how the greenhouse gases carbon dioxide and methane cause global warming, limited to:
(a) the absorption, reflection and emission of thermal energy
(b) reducing thermal energy loss to space - Explain how oxides of nitrogen form in car engines and describe their removal by catalytic converters, e.g. 2CO + 2NO → 2CO2 + N2
State the symbol equation for photosynthesis, 6CO2 + 6H2O → C6H12O6 + 6O2
Composition of air and its Pollutants
Composition of air and its Pollutants
Clean, dry air is a mixture of gases in specific proportions: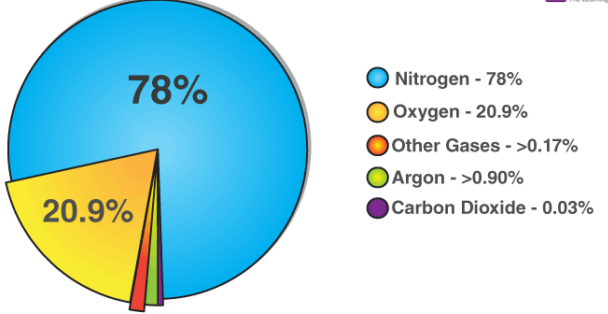
Nitrogen (\( \text{N}_2 \)) – about 78%
- Nitrogen is the most abundant gas in the atmosphere.
- It is chemically unreactive under normal conditions because of its very strong triple covalent bond.
- It acts as a diluent, reducing the reactivity of oxygen and helping maintain a stable atmosphere.
- Nitrogen is essential for the formation of proteins in plants and animals, but must first be converted into compounds such as nitrates, ammonium salts, or urea.
Oxygen (\( \text{O}_2 \)) – about 21%
- Oxygen is the second most abundant gas.
- It is highly reactive and supports combustion, respiration, and many oxidation reactions.
- Oxygen is vital for aerobic respiration in living organisms and is also released during photosynthesis.
Noble gases – less than 1%
- Includes argon (most abundant of the noble gases in air), neon, helium, krypton, and xenon.
- These gases are chemically unreactive due to their stable electronic configurations.
- Argon is about 0.9% of air and is used in applications like providing an inert atmosphere in welding and inside light bulbs.
Carbon dioxide (\( \text{CO}_2 \)) – about 0.04%
- Present in a small proportion but very important for life and climate.
- Used by plants during photosynthesis to make glucose and oxygen.
- Acts as a greenhouse gas, trapping heat and helping regulate Earth’s temperature.
Key note:
The composition above is for clean, dry air. In real atmospheric conditions, variable amounts of water vapour are also present (up to around 4%), which affect weather and climate but are excluded in this standard composition.
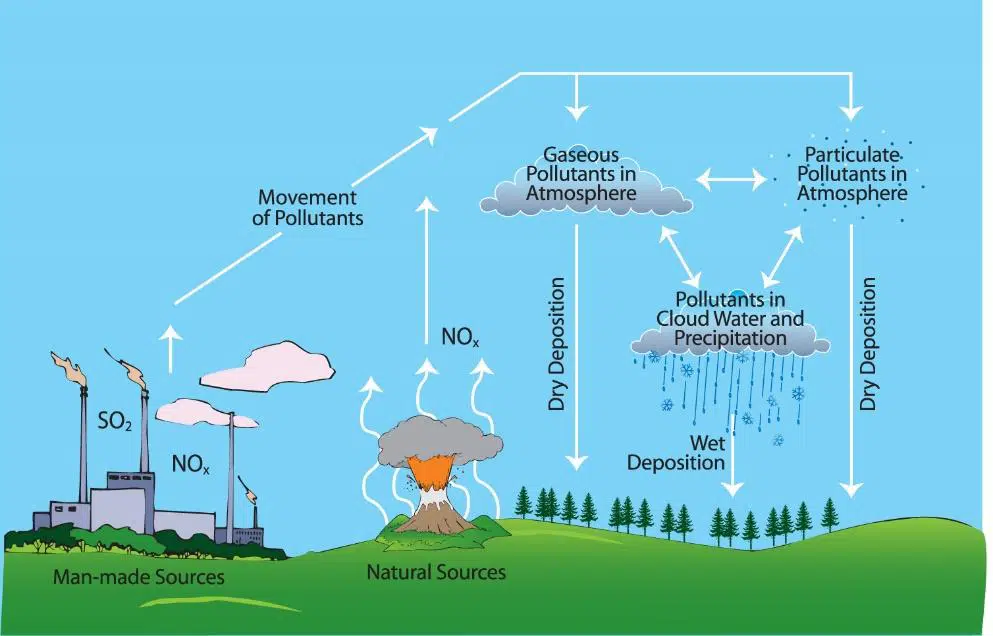
Air pollutants: sources and adverse effects
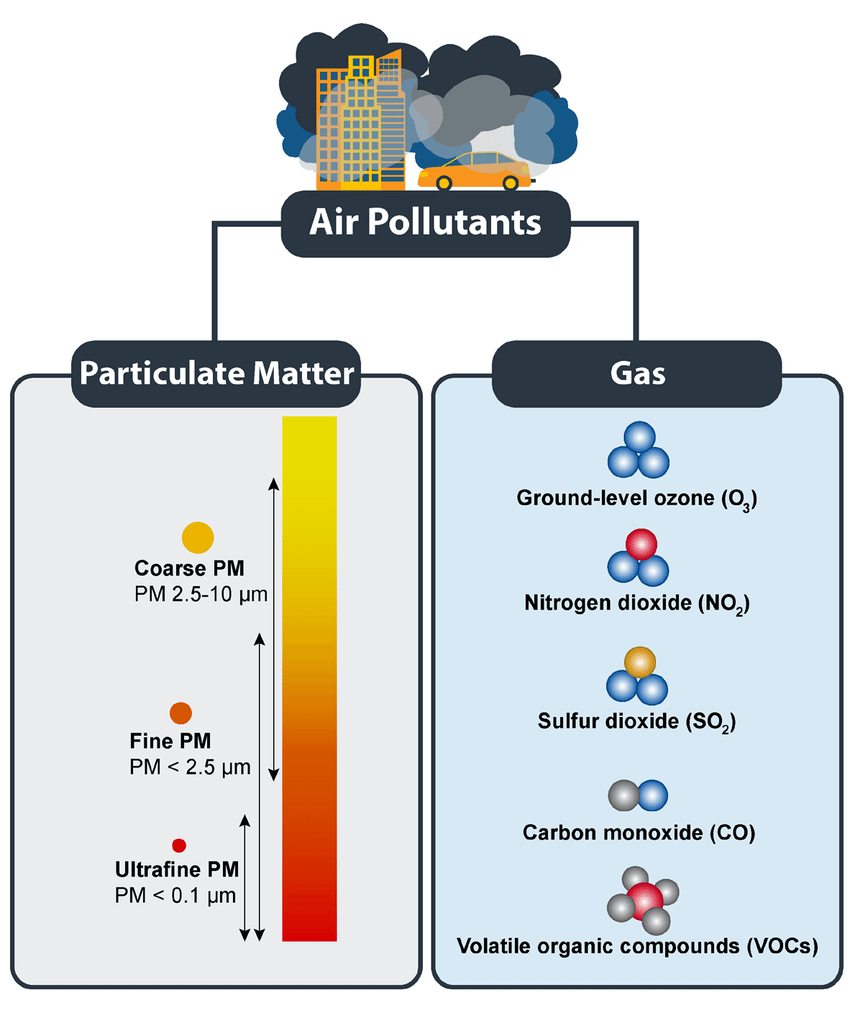
The main air pollutants, their sources, and harmful effects are:
Carbon dioxide (\( \text{CO}_2 \))
- Source: Produced by complete combustion of carbon-containing fuels (coal, oil, natural gas, petrol, diesel).
- Effect: Greenhouse gas that traps heat in the atmosphere, causing global warming and climate change (rising sea levels, extreme weather, melting ice).
Carbon monoxide (\( \text{CO} \))
- Source: Formed during incomplete combustion of fuels when oxygen supply is limited (e.g. car engines, poorly ventilated stoves).
- Effect: A colourless, odourless but highly toxic gas; binds with haemoglobin to form carboxyhaemoglobin, reducing oxygen transport in blood → dizziness, unconsciousness, or death.
Particulates (soot, unburnt carbon)
- Source: Released during incomplete combustion of fuels (diesel engines, coal burning, open fires).
- Effect: Cause respiratory diseases such as asthma, bronchitis, and lung cancer. Contribute to smog and reduce visibility. Darken ice surfaces, increasing melting and worsening climate change.
Methane (\( \text{CH}_4 \))
- Source: Produced by decomposition of vegetation in swamps/landfills, released during digestion in ruminant animals (cows, sheep), from rice paddies, and natural gas leaks.
- Effect: Very powerful greenhouse gas (much stronger than \( \text{CO}_2 \)); causes global warming and climate change. Also contributes to formation of ground-level ozone in smog.
Oxides of nitrogen (\( \text{NO}, \text{NO}_2 \))
- Source: Formed inside car engines at high temperatures when nitrogen and oxygen from air react.
- Effect: Cause acid rain (damages plants, corrodes buildings, kills fish), contribute to photochemical smog (eye irritation, poor visibility), and worsen respiratory illnesses like asthma.
Sulfur dioxide (\( \text{SO}_2 \))
- Source: Produced when fossil fuels containing sulfur compounds (coal, crude oil) are burned in power stations, factories, or ships.
- Effect: Causes acid rain (damages forests, erodes buildings/statues, acidifies lakes killing aquatic life). Irritates lungs and increases risk of respiratory problems.
Key note:
Most pollutants come from the combustion of fuels, especially in vehicles, industries, and power plants, and their effects are both local (health, smog, acid rain) and global (climate change, global warming).
Formation and removal of oxides of nitrogen in car engines
Oxides of nitrogen, commonly written as \( \text{NO}_x \) (mainly nitrogen monoxide \( \text{NO} \) and nitrogen dioxide \( \text{NO}_2 \)), are harmful air pollutants formed in car engines and power stations.
Formation of oxides of nitrogen:
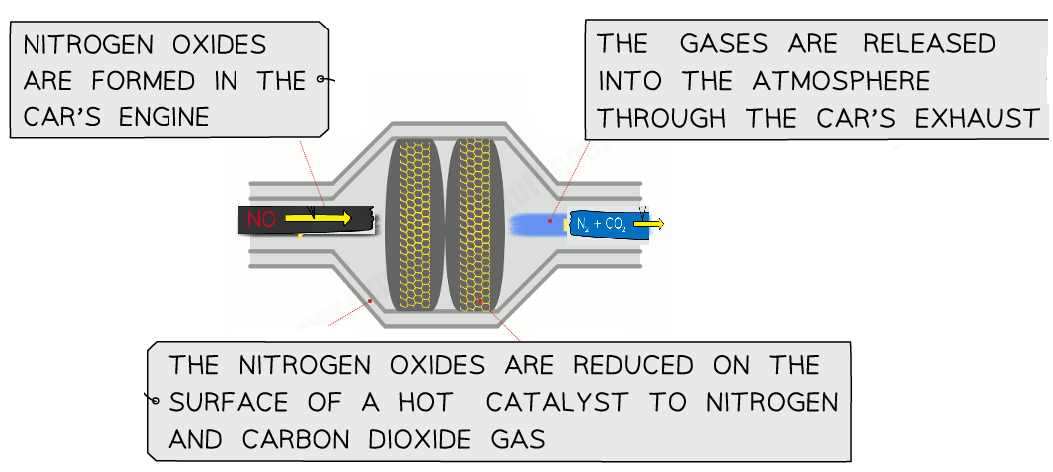
- Car engines operate at very high temperatures during fuel combustion.
- Normally, nitrogen \( \text{N}_2 \) and oxygen \( \text{O}_2 \) from the air do not react because both are stable molecules.
- However, at these extreme temperatures, the strong triple bond in nitrogen can break, allowing nitrogen and oxygen to combine and form nitrogen monoxide \( \text{NO} \).
- Nitrogen monoxide can then react further with oxygen in the atmosphere to form nitrogen dioxide \( \text{NO}_2 \).
Example reactions:
\( \text{N}_2 + \text{O}_2 \rightarrow 2\text{NO} \)
\( 2\text{NO} + \text{O}_2 \rightarrow 2\text{NO}_2 \)
Removal of oxides of nitrogen by catalytic converters:
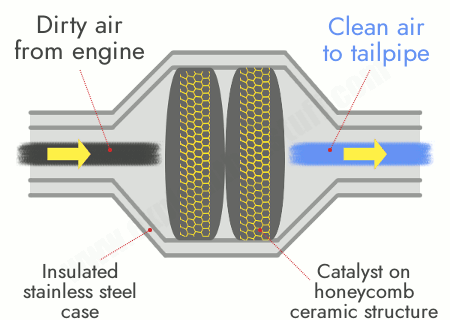
- Cars are fitted with catalytic converters in their exhaust systems to reduce harmful emissions.
- The catalyst is usually made of platinum, rhodium, or palladium coated on a ceramic surface with a honeycomb structure (to increase surface area).
- Inside the catalytic converter, nitrogen oxides are reduced back to nitrogen gas, while carbon monoxide is oxidised to carbon dioxide.
Key reaction in a catalytic converter:
\( 2\text{CO} + 2\text{NO} \rightarrow 2\text{CO}_2 + \text{N}_2 \)
Example
Why is carbon monoxide (\( \text{CO} \)) considered more dangerous to humans than carbon dioxide (\( \text{CO}_2 \)) even though both are combustion products?
▶️Answer/Explanation
Carbon monoxide: It is formed by incomplete combustion of fuels. It is colourless and odourless, but highly toxic because it binds strongly with haemoglobin in the blood to form carboxyhaemoglobin. This prevents oxygen from binding, reducing oxygen transport in the body. The result is suffocation symptoms like dizziness, unconsciousness, or even death.
Carbon dioxide: It is formed by complete combustion of fuels. It is not directly toxic in normal concentrations but contributes to global warming as a greenhouse gas.
Final Answer: \( \text{CO} \) is more dangerous to humans than \( \text{CO}_2 \) because it directly interferes with oxygen transport in blood.
Example
Explain how oxides of nitrogen (\( \text{NO}_x \)) are formed in car engines and how catalytic converters help remove them.
▶️Answer/Explanation
Formation of \( \text{NO}_x \): At the very high temperatures inside a car engine, nitrogen and oxygen from the air react:
\( \text{N}_2 + \text{O}_2 \rightarrow 2\text{NO} \)
The nitrogen monoxide formed can react further with oxygen to produce nitrogen dioxide:
\( 2\text{NO} + \text{O}_2 \rightarrow 2\text{NO}_2 \)
Both \( \text{NO} \) and \( \text{NO}_2 \) contribute to acid rain and photochemical smog.
Removal in catalytic converters: Exhaust gases pass over a platinum/rhodium/palladium catalyst. Harmful gases are converted into harmless ones:
\( 2\text{CO} + 2\text{NO} \rightarrow 2\text{CO}_2 + \text{N}_2 \)
Final Answer: \( \text{NO}_x \) gases are formed in car engines due to high temperatures, and catalytic converters remove them by reducing them back to nitrogen gas while oxidising carbon monoxide to carbon dioxide.
Strategies to reduce environmental issues
Strategies to reduce environmental issues
Different strategies are used to reduce the harmful effects of pollutants and their impact on the environment:
Climate change (caused by carbon dioxide and methane)
Problem:
Excess greenhouse gases trap too much heat, causing global warming and climate change.
Strategies:
- Planting more trees to absorb \( \text{CO}_2 \) during photosynthesis.
- Reducing livestock farming to decrease methane emissions.
- Decreasing the use of fossil fuels by switching to cleaner energy.
- Increasing the use of hydrogen fuel which produces only water as a product.
- Expanding renewable energy sources such as wind, solar, and hydroelectric power.
Acid rain (caused by sulfur dioxide and oxides of nitrogen)
Problem:
Acidic gases dissolve in rainwater to form acid rain, which damages ecosystems, buildings, and aquatic life.
Strategies:
- Using catalytic converters in vehicles to remove harmful gases like \( \text{NO}_x \) and \( \text{CO} \).
- Burning low-sulfur fuels to reduce sulfur dioxide emissions.
- Installing flue gas desulfurisation systems in power stations, where waste gases are treated with calcium oxide to neutralise sulfur dioxide and form harmless calcium salts.
Example
Explain two different strategies used to reduce the effects of:
- Climate change caused by greenhouse gases
- Acid rain caused by sulfur dioxide and oxides of nitrogen
▶️Answer/Explanation
Climate change:
One strategy is planting more trees, which absorb \( \text{CO}_2 \) during photosynthesis, thereby reducing the amount of greenhouse gas in the atmosphere. Another strategy is switching to renewable energy sources such as wind and solar instead of fossil fuels, which lowers both carbon dioxide and methane emissions.
Acid rain:
One strategy is fitting vehicles with catalytic converters. These devices reduce harmful nitrogen oxides (\( \text{NO}_x \)) to nitrogen gas, and oxidise carbon monoxide to carbon dioxide, lowering acid rain precursors. Another strategy is installing flue gas desulfurisation units in power stations, where waste gases containing \( \text{SO}_2 \) are treated with calcium oxide to form harmless calcium salts, preventing sulfur dioxide from escaping into the atmosphere.
Final Answer: Climate change is tackled by reducing greenhouse gases (e.g., planting trees, renewable energy), while acid rain is reduced by controlling emissions at source (e.g., catalytic converters, flue gas desulfurisation).
Photosynthesis
Photosynthesis
Photosynthesis is the fundamental process by which green plants, algae, and some bacteria trap light energy from the sun and convert it into chemical energy stored in glucose. This process is essential for life on Earth because it provides food and releases oxygen into the atmosphere.
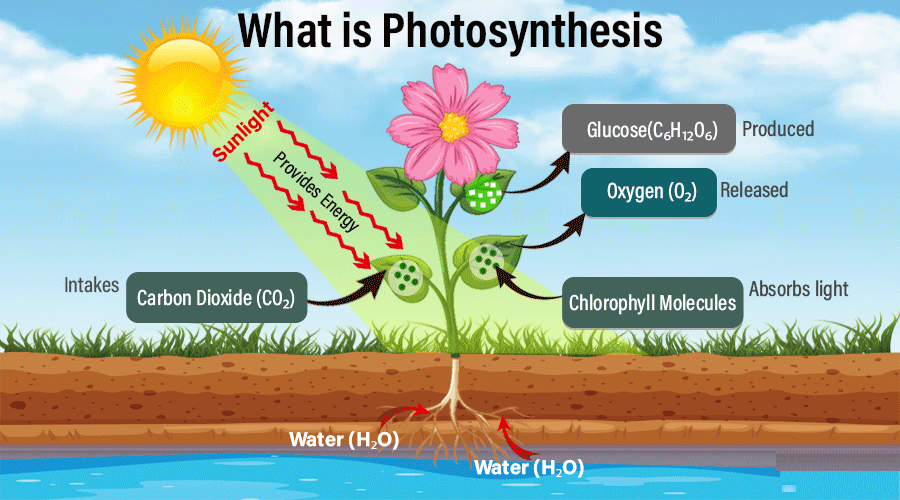
Conditions required:
- Carbon dioxide: Plants take in \( \text{CO}_2 \) from the atmosphere through small pores in their leaves called stomata. It provides the carbon atoms needed to build glucose molecules.
- Water: Absorbed by the roots from the soil and transported through the xylem vessels to the leaves. It provides hydrogen atoms and also releases oxygen during the process.
- Light energy: The source of energy that drives the reaction, mainly sunlight.
- Chlorophyll: A green pigment found in chloroplasts of plant cells that absorbs sunlight and converts it into chemical energy for the process.
Word equation:
carbon dioxide + water → glucose + oxygen
Symbol equation:
\( 6\text{CO}_2 + 6\text{H}_2\text{O} \xrightarrow{\text{light, chlorophyll}} \text{C}_6\text{H}_{12}\text{O}_6 + 6\text{O}_2 \)
Explanation of each part:
- \( 6\text{CO}_2 \): Six molecules of carbon dioxide provide the carbon atoms needed to form glucose.
- \( 6\text{H}_2\text{O} \): Six molecules of water provide hydrogen atoms and release oxygen in the process.
- \( \text{C}_6\text{H}_{12}\text{O}_6 \): One molecule of glucose is produced, storing energy in chemical bonds.
- \( 6\text{O}_2 \): Six molecules of oxygen are released as a by-product into the atmosphere.
Process description:
During photosynthesis, light energy absorbed by chlorophyll is used to split water molecules into hydrogen and oxygen. The hydrogen is combined with carbon dioxide to produce glucose, while oxygen is released as a by-product. The glucose formed is an energy-rich compound that can be: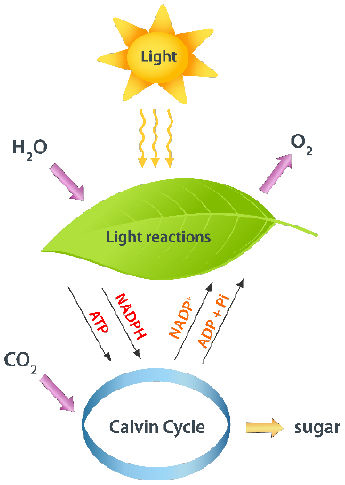
- Used immediately in respiration to release energy.
- Converted into starch for storage in leaves, stems, or roots.
- Used to form cellulose, proteins, and fats needed for plant growth and development.
Products formed:
- Glucose: The main sugar formed during photosynthesis. It stores chemical energy from sunlight and is used by the plant for respiration, growth, and storage as starch.
- Oxygen: A by-product released into the atmosphere through the stomata. It is vital for the respiration of animals and humans.
Importance of photosynthesis:
- It produces glucose, which is the basic food source for plants and indirectly for animals.
- It releases oxygen into the atmosphere, which is essential for aerobic respiration in animals and humans.
- It helps regulate atmospheric levels of carbon dioxide by absorbing it, thereby reducing the greenhouse effect.
- It is the starting point of most food chains, making it the foundation of nearly all ecosystems.
Example
State the word equation for photosynthesis and explain how light intensity and carbon dioxide concentration affect the rate of photosynthesis.
▶️Answer/Explanation
Word equation for photosynthesis:
Carbon dioxide + Water → Glucose + Oxygen
(In symbols: \( 6\text{CO}_2 + 6\text{H}_2\text{O} \xrightarrow{\text{light/chlorophyll}} \text{C}_6\text{H}_{12}\text{O}_6 + 6\text{O}_2 \))
Effect of light intensity:
As light intensity increases, the rate of photosynthesis increases because more light energy is available to split water molecules and drive the reactions. However, beyond a certain point, the rate levels off as another factor becomes limiting (such as carbon dioxide or temperature).
Effect of carbon dioxide concentration:
As carbon dioxide concentration increases, the rate of photosynthesis increases because more raw material is available for glucose production. Beyond a certain level, the rate stops increasing since another factor (like light or temperature) becomes limiting.
Final Answer: Photosynthesis converts carbon dioxide and water into glucose and oxygen using light energy. Both light intensity and carbon dioxide concentration increase the rate until another limiting factor takes over.
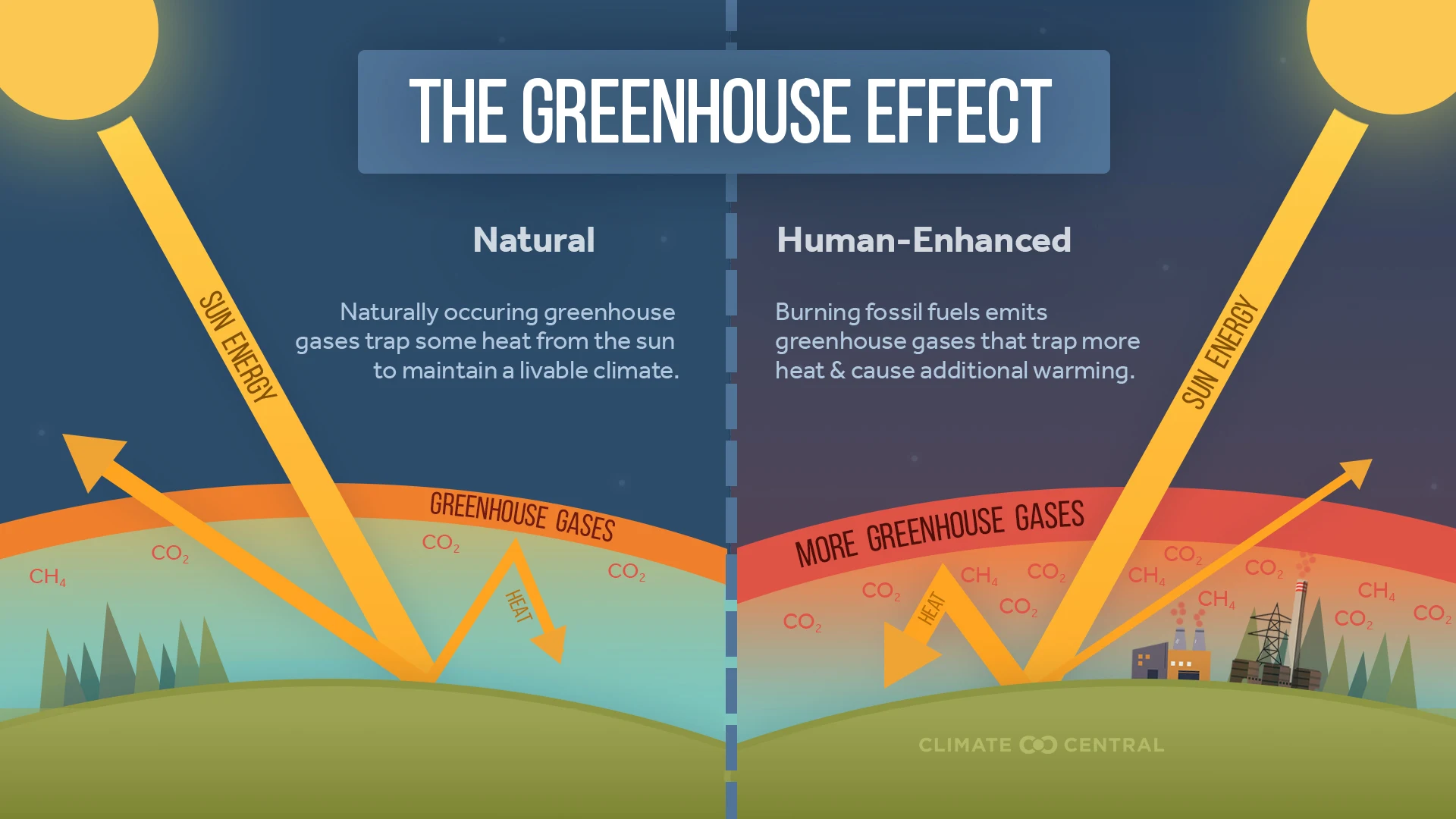
How greenhouse gases cause global warming
How greenhouse gases cause global warming
Greenhouse gases such as carbon dioxide \( \text{CO}_2 \) and methane \( \text{CH}_4 \) play a major role in global warming by trapping heat in the Earth’s atmosphere. This natural process is called the greenhouse effect, but when levels of these gases rise due to human activity, the effect intensifies and leads to climate change.
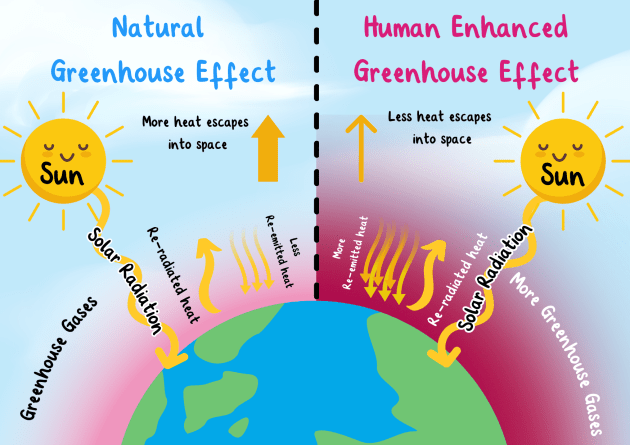
Step-by-step explanation of the process:
- Incoming short-wave radiation from the sun passes through the atmosphere and reaches the Earth’s surface.
- The Earth absorbs this energy, warms up, and then re-emits it as longer-wave infrared (thermal) radiation.
- Greenhouse gases such as \( \text{CO}_2 \) and \( \text{CH}_4 \) absorb much of this outgoing infrared radiation.
- Instead of allowing the heat to escape into space, these gases re-radiate the thermal energy back towards the Earth’s surface.
- This process reduces the loss of thermal energy to space, effectively trapping heat and causing the Earth’s average temperature to rise.
Key consequences:
- Higher global temperatures lead to melting ice caps, rising sea levels, and changes in weather patterns.
- Increased frequency of extreme events such as droughts, floods, and stronger storms.
- Disruption of ecosystems and threats to biodiversity.
Example
Explain how greenhouse gases such as carbon dioxide and methane cause global warming.
▶️Answer/Explanation
Step 1: Absorption of energy from the Sun
The Sun’s energy reaches the Earth in the form of short-wave radiation (mainly visible light). The Earth’s surface absorbs this energy and warms up.
Step 2: Re-radiation of energy
The warmed Earth re-radiates energy back into the atmosphere as long-wave infrared radiation (heat).
Step 3: Trapping by greenhouse gases
Greenhouse gases such as carbon dioxide, methane, and water vapour absorb this infrared radiation and re-emit it in all directions, including back towards Earth.
Step 4: Result – global warming
This “trapping” of heat keeps the Earth warmer than it would be naturally. Increasing concentrations of greenhouse gases intensify this effect, leading to global warming, melting ice caps, rising sea levels, and extreme weather events.
Final Answer: Greenhouse gases cause global warming by absorbing outgoing infrared radiation and re-emitting it, preventing heat from escaping into space and raising Earth’s temperature.
