Alcohols- CIE iGCSE Chemistry Notes - New Syllabus
Alcohols for iGCSE Chemistry Notes
Core Syllabus
- Describe the manufacture of ethanol by:
(a) fermentation of aqueous glucose at 25–35 °C in the presence of yeast and in the absence of oxygen
(b) catalytic addition of steam to ethene at 300 °C and 6000 kPa / 60 atm in the presence of an acid catalyst - Describe the combustion of ethanol
- State the uses of ethanol as:
(a) a solvent
(b) a fuel
Supplement Syllabus
- Describe the advantages and disadvantages of the manufacture of ethanol by:
(a) fermentation
(b) catalytic addition of steam to ethene
Manufacture of Ethanol
Manufacture of Ethanol
(a) Fermentation of aqueous glucose 
- Ethanol can be made by fermentation of glucose.
- Glucose is obtained from sugar cane, maize, or other carbohydrate crops.
- The process takes place at 25-35 °C.
- Yeast is used as a natural catalyst because it contains enzymes.
- The reaction must occur in the absence of oxygen (anaerobic conditions).
- The equation is:
\( \text{C}_6\text{H}_{12}\text{O}_6 \rightarrow 2\text{C}_2\text{H}_5\text{OH} + 2\text{CO}_2 \). - Ethanol is collected from the mixture by fractional distillation since the solution produced is dilute.
Advantages:
- Uses renewable resources (sugar cane, maize, etc.).
- Requires relatively low temperature (25-35 °C), so less energy is needed.
- Can be carried out with simple technology in smallscale or rural areas.
Disadvantages:
- Slow process compared to industrial catalytic methods.
- Produces dilute ethanol solution that must be purified by fractional distillation (energy costly).
- Requires large areas of farmland, which may compete with food production.
- Carbon dioxide is produced, contributing to greenhouse gases.
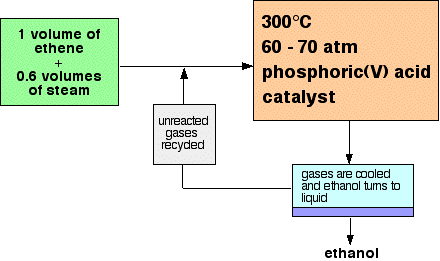
(b) Catalytic addition of steam to ethene
- Ethanol can also be manufactured from ethene obtained from petroleum cracking.

- Ethene is reacted with steam under controlled conditions.
- Temperature used is about 300 °C.
- Pressure used is about 6000 kPa (≈60 atm).
- An acid catalyst such as concentrated phosphoric acid is used.
- The reaction is an addition reaction across the C=C double bond.
- The equation is:
\( \text{C}_2\text{H}_4 + \text{H}_2\text{O} \rightarrow \text{C}_2\text{H}_5\text{OH} \).
Advantages:
- Fast and continuous process, suitable for large-scale industrial production.
- Produces pure ethanol directly, no need for fractional distillation.
- More efficient and less labor-intensive.
Disadvantages:
- Uses ethene, which is obtained from petroleum – a non-renewable resource.
- Requires very high temperature (≈300 °C) and pressure (≈60 atm), so high energy costs.
- Dependence on crude oil supply and price fluctuations.
Example
Ethanol can be produced by fermentation of glucose or by catalytic hydration of ethene. Compare the two processes in terms of raw materials, conditions, catalysts, and yield. Write balanced equations for both methods.
▶️Answer/Explanation
Fermentation of glucose:
- Raw material: glucose from sugar or starch crops
- Conditions: 25-35 °C, absence of oxygen
- Catalyst: enzymes in yeast
- Equation: \( \text{C}_6\text{H}_{12}\text{O}_6 \rightarrow 2\text{C}_2\text{H}_5\text{OH} + 2\text{CO}_2 \)
- Yield: moderate, solution of ethanol obtained (about 15%)
Catalytic hydration of ethene:
- Raw material: ethene from cracking petroleum
- Conditions: 300 °C, 60 atm
- Catalyst: phosphoric acid on silica
- Equation: \( \text{C}_2\text{H}_4 + \text{H}_2\text{O} \rightarrow \text{C}_2\text{H}_5\text{OH} \)
- Yield: continuous, pure ethanol produced directly
Comparison:
- Fermentation uses renewable resources but is slow and batch-based.
- Catalytic hydration is faster, continuous, and gives pure ethanol, but depends on non-renewable crude oil.
Combustion of Ethanol
Combustion of Ethanol
Ethanol burns readily in oxygen because it is a hydrocarbon-based fuel containing carbon, hydrogen, and oxygen atoms. The combustion is an exothermic reaction, releasing a large amount of energy as heat and light.
Complete combustion:
When there is a plentiful supply of oxygen, ethanol undergoes complete combustion to form carbon dioxide and water:
\( \text{C}_2\text{H}_5\text{OH} + 3\text{O}_2 \rightarrow 2\text{CO}_2 + 3\text{H}_2\text{O} \)
Incomplete combustion:
If oxygen is limited, incomplete combustion occurs. This produces carbon monoxide (a poisonous gas) and/or carbon (soot) along with water:
\( \text{C}_2\text{H}_5\text{OH} + 2\text{O}_2 \rightarrow 2\text{CO} + 3\text{H}_2\text{O} \)
\( \text{C}_2\text{H}_5\text{OH} + \text{O}_2 \rightarrow 2\text{C} + 3\text{H}_2\text{O} \)
Key features of ethanol combustion:
- Ethanol burns with a clean, pale blue flame.
- Produces significant energy, making it useful as a biofuel and in alcoholic spirit burners.
- Complete combustion is preferred for maximum energy release and minimal pollution.
- Incomplete combustion reduces efficiency and produces harmful carbon monoxide.
Uses of Ethanol
Uses of Ethanol
Ethanol is an important organic compound with a wide range of practical applications due to its chemical properties: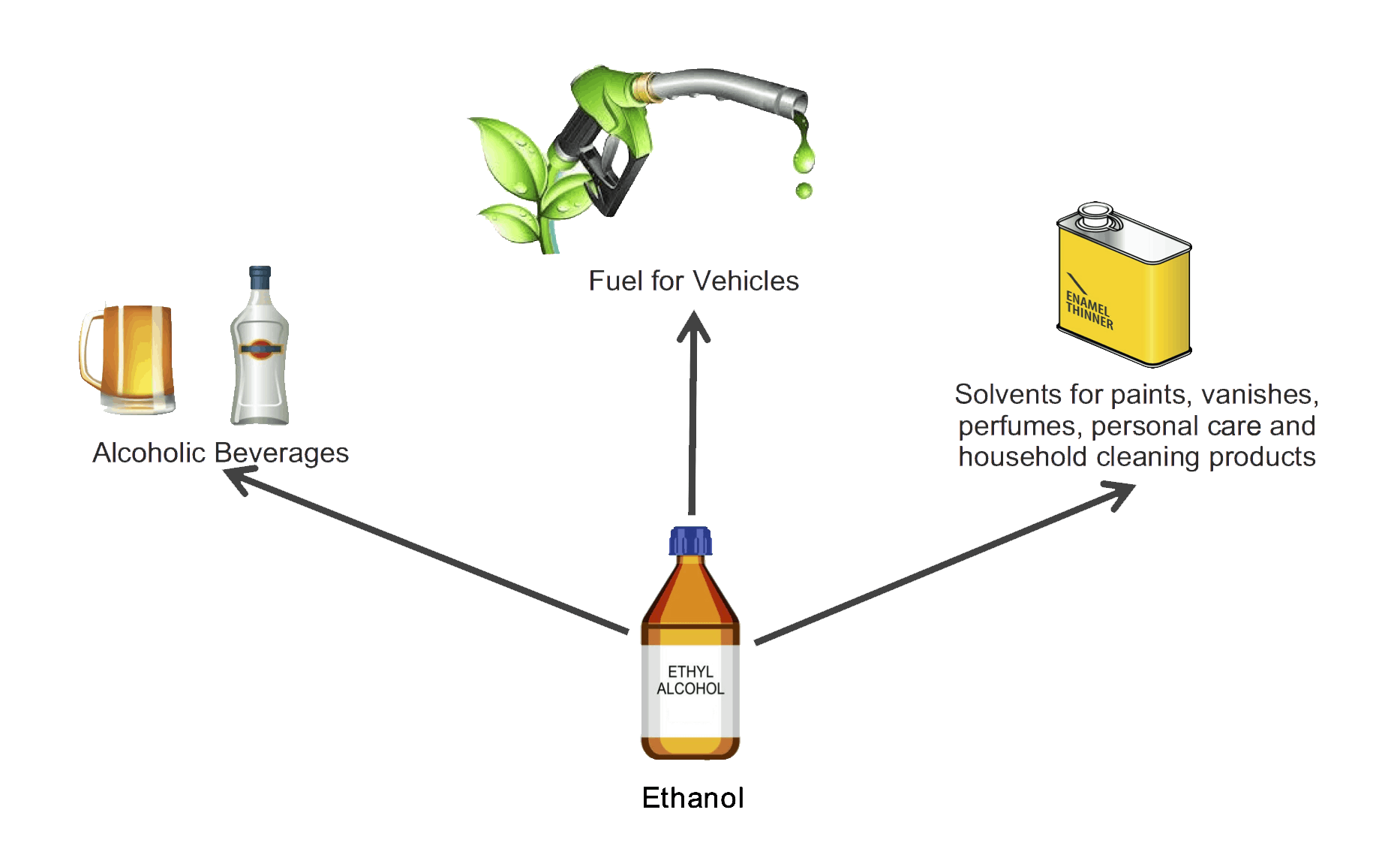
(a) Ethanol as a Solvent
- Ethanol is a good solvent for many organic compounds that do not dissolve in water, such as oils, fats, resins, and perfumes.
- It is commonly used in the manufacture of cosmetics, perfumes, deodorants, medicines, and varnishes.
- Ethanol is also used in alcoholic beverages, where it acts as the solvent for flavors and aromas.
(b) Ethanol as a Fuel
- Ethanol burns cleanly with a pale blue flame, releasing a large amount of heat energy.
- It is used as a biofuel (renewable fuel made from plant material, such as sugarcane or maize).
- Ethanol is often blended with petrol to make gasohol, which reduces dependence on nonrenewable fossil fuels.
- As a fuel, ethanol helps to reduce greenhouse gas emissions compared to pure petrol, but it still contributes some carbon dioxide when burned.
Example
A factory is deciding how to use ethanol that it produces. Suggest two different uses of ethanol, giving one chemical property or advantage for each use.
▶️Answer/Explanation
Use 1: As a solvent
Ethanol dissolves both water-soluble and organic compounds because it has a polar -OH group and a non-polar hydrocarbon chain. Example: It is used in medicines, perfumes, and inks as a solvent for organic substances.
Use 2: As a fuel
Ethanol burns cleanly in oxygen to give carbon dioxide and water, releasing energy. Equation: \( \text{C}_2\text{H}_5\text{OH} + 3\text{O}_2 \rightarrow 2\text{CO}_2 + 3\text{H}_2\text{O} \). Example: It is used in cars as a renewable fuel when blended with petrol (bioethanol fuel).
Key point: One use depends on ethanol’s solvent property, the other on its combustion property.