Alkanes- CIE iGCSE Chemistry Notes - New Syllabus
Alkanes for iGCSE Chemistry Notes
Core Syllabus
- State that the bonding in alkanes is single covalent and that alkanes are saturated hydrocarbons
- Describe the properties of alkanes as being generally unreactive, except in terms of combustion and substitution by chlorine
Supplement Syllabus
- State that in a substitution reaction one atom or group of atoms is replaced by another atom or group of atoms
- Describe the substitution reaction of alkanes with chlorine as a photochemical reaction, with ultraviolet light providing the activation energy, Ea, and draw the structural or displayed formulae of the products, limited to monosubstitution
Alkanes
Bonding and Saturation in Alkanes
Alkanes are a homologous series of hydrocarbons.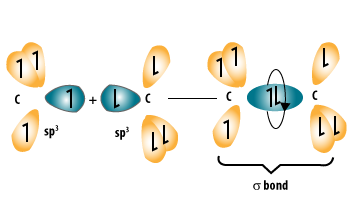
- They contain only single covalent bonds between carbon atoms.
- Each carbon atom in an alkane forms four single covalent bonds (C–C or C–H).
- Each hydrogen atom in an alkane forms one single covalent bond with carbon.
- Because they contain only single bonds, alkanes are described as saturated hydrocarbons.
- Saturated means that no more hydrogen atoms can be added without breaking existing bonds.
- The general formula of alkanes is \( \text{C}_n\text{H}_{2n+2} \).
Example
State whether ethane is a saturated or unsaturated hydrocarbon, and explain your answer.
▶️Answer/Explanation
Ethane has the formula \( \text{C}_2\text{H}_6 \). All bonds are single covalent bonds (C–C and C–H). This means it cannot accept additional hydrogen atoms without breaking bonds. Therefore, ethane is a saturated hydrocarbon.
Properties of Alkanes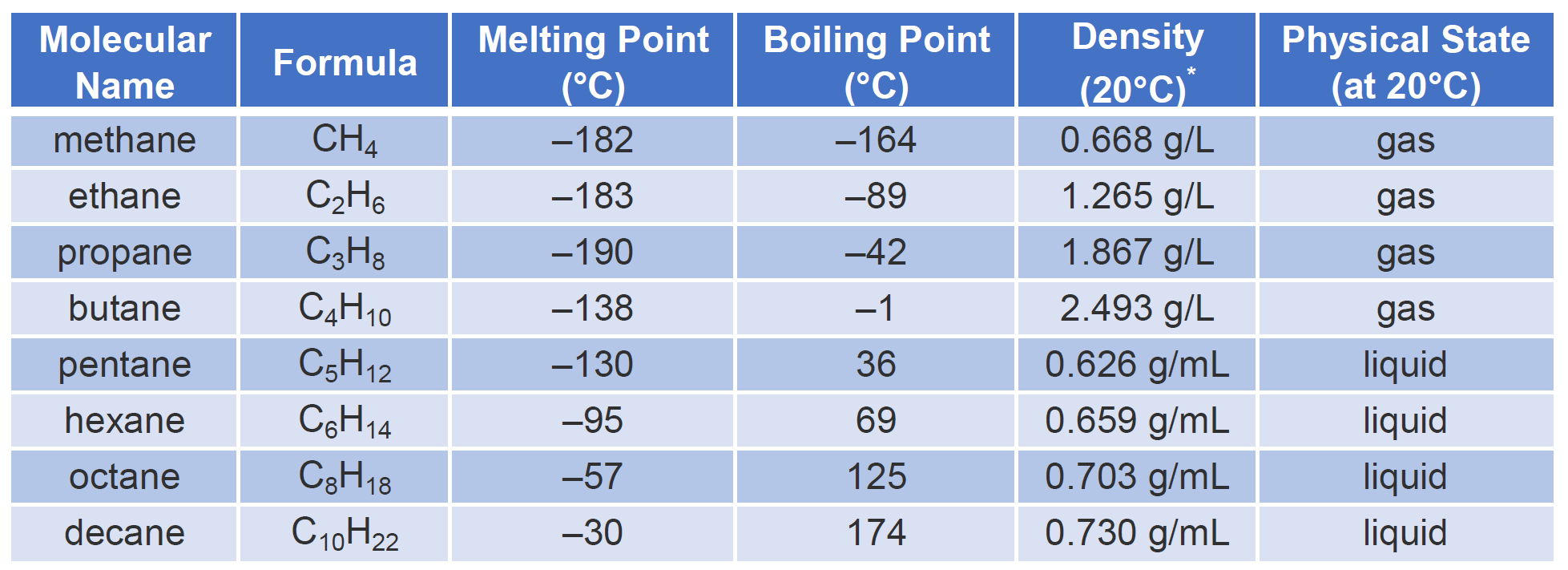
- Alkanes are generally unreactive compared to other organic compounds.
- This is because the C–C and C–H bonds are strong covalent bonds.
- The bonds in alkanes are non-polar or only slightly polar, so they do not attract many reagents.
- As a result, alkanes do not usually react with acids, alkalis, or oxidising agents under normal conditions.
- However, alkanes undergo combustion reactions in the presence of oxygen.
- Complete combustion produces carbon dioxide and water: \( \text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} \).
- Incomplete combustion occurs if oxygen is limited, producing carbon monoxide and water, or even carbon (soot).
- Alkanes can also undergo substitution reactions, where hydrogen atoms are replaced by other atoms such as chlorine.
- Substitution reactions of alkanes require ultraviolet light to start the process.
Example
Explain why methane is considered generally unreactive, and give one condition where it does react.
▶️Answer/Explanation
Methane contains only strong C–H and C–C single covalent bonds, which are difficult to break. This makes it generally unreactive under normal conditions. However, methane reacts readily with oxygen in a combustion reaction to produce carbon dioxide and water when burned in air.
Substitution Reactions in Alkanes
Substitution Reactions in Alkanes 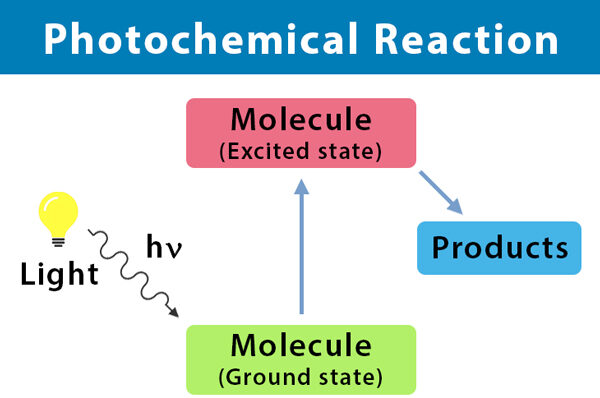
- A substitution reaction is a type of chemical reaction where one atom or group of atoms in a molecule is replaced by another atom or group of atoms.
- In the case of alkanes, substitution reactions usually occur with halogens such as chlorine or bromine.
- For example, in methane (\( \text{CH}_4 \)), one hydrogen atom can be replaced by a chlorine atom to form chloromethane (\( \text{CH}_3\text{Cl} \)).
- The hydrogen atom that is substituted is released as hydrogen chloride (\( \text{HCl} \)).
- The general pattern is: \( \text{R–H} + \text{Cl}_2 \rightarrow \text{R–Cl} + \text{HCl} \) where R–H represents an alkane.
- This type of reaction is typical of saturated hydrocarbons under the right conditions.
Substitution Reaction of Alkanes with Chlorine (Photochemical Reaction)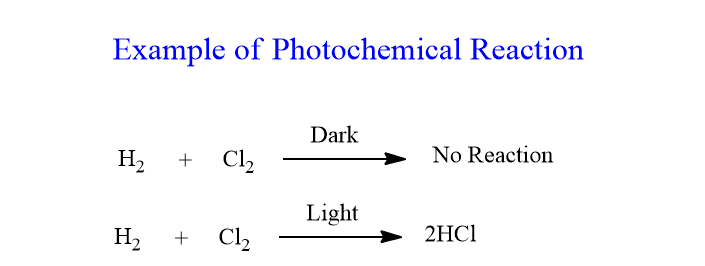
- Alkanes generally do not react with chlorine in the dark or at room temperature because they are relatively unreactive.
- However, in the presence of ultraviolet (UV) light, alkanes can undergo a substitution reaction with chlorine.
- This reaction is called a photochemical reaction because it requires light (photons) to proceed.
- The UV light provides the activation energy (Ea) needed to break the \( \text{Cl–Cl} \) bond, producing chlorine free radicals (\( \text{Cl} \cdot \)).
- The chlorine radicals are highly reactive and substitute hydrogen atoms in the alkane, producing a halogenoalkane (alkyl halide) and hydrogen chloride.
- The substitution can occur multiple times, replacing more than one hydrogen, but at IGCSE level, focus is limited to monosubstitution (only one hydrogen atom replaced).
- General reaction for monosubstitution: \( \text{CH}_4 + \text{Cl}_2 \xrightarrow{\text{UV light}} \text{CH}_3\text{Cl} + \text{HCl} \)
- The reaction proceeds through a free radical mechanism (initiation, propagation, termination), but detailed steps are usually studied at higher levels.
Example
Explain why UV light is required for the substitution reaction between methane and chlorine, and write the product formed.
▶️Answer/Explanation
UV light is needed to supply the activation energy (Ea) required to break the \( \text{Cl–Cl} \) bond in chlorine molecules, forming chlorine radicals. These radicals are highly reactive and replace hydrogen atoms in methane. The reaction is: \( \text{CH}_4 + \text{Cl}_2 \xrightarrow{\text{UV light}} \text{CH}_3\text{Cl} + \text{HCl} \). The product formed is chloromethane along with hydrogen chloride.
