Arrangement of elements- CIE iGCSE Chemistry Notes - New Syllabus
Arrangement of elements for iGCSE Chemistry Notes
Core Syllabus
- Describe the Periodic Table as an arrangement of elements in periods and groups and in order of increasing proton number / atomic number
- Describe the change from metallic to non-metallic character across a period
- Describe the relationship between group number and the charge of the ions formed from elements in that group
- Explain similarities in the chemical properties of elements in the same group of the Periodic Table in terms of their electronic configuration
- Explain how the position of an element in the Periodic Table can be used to predict its properties
Supplement Syllabus
- Identify trends in groups, given information about the elements
The Periodic Table
The Periodic Table
Structure of the Periodic Table
- The Periodic Table is a chart that organises all known chemical elements in a systematic way based on their properties and atomic structure.
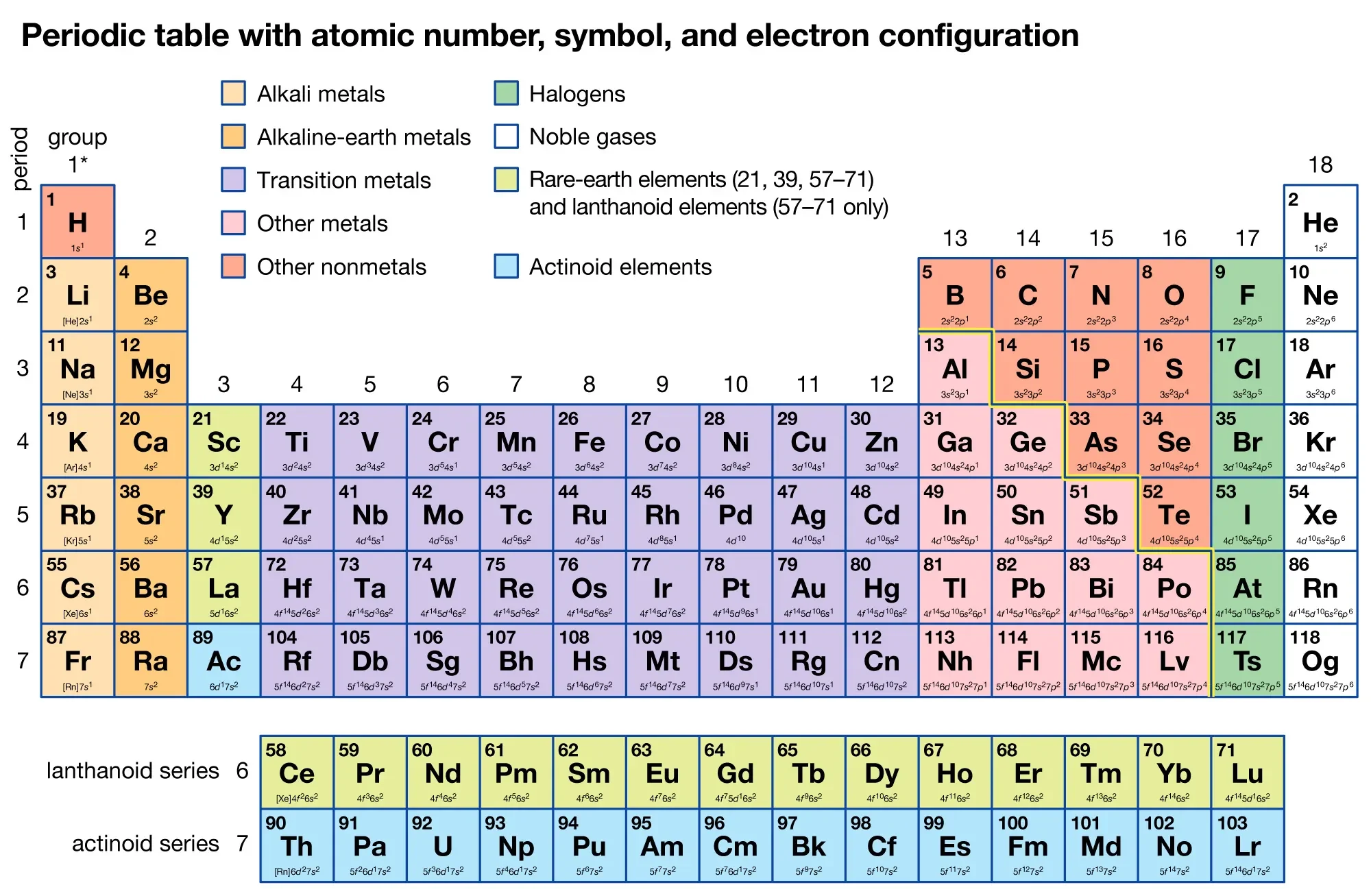
- Elements are arranged in rows called periods and columns called groups.
- Each element is positioned in order of increasing proton number (also called atomic number), which is the number of protons in the nucleus of its atoms.
Periods (horizontal rows)
- There are 7 main periods in the modern Periodic Table.
- As you move across a period from left to right, the proton number increases by 1 for each successive element.
- All elements in the same period have the same number of electron shells, but their number of outer electrons changes across the row.
- Physical and chemical properties change gradually across a period due to changes in electron configuration.
Groups (vertical columns)
- There are 18 groups in the modern Periodic Table.
- All elements in the same group have the same number of outer (valence) electrons, giving them similar chemical properties.
- Group numbers for the main-group elements (Groups 1–2 and 13–18) indicate the number of valence electrons.
- Elements in a group show trends in reactivity and other properties as you move down the group.
Arrangement by increasing proton number
- The proton number uniquely identifies an element and determines its position in the table.
- Because the number of protons equals the number of electrons in a neutral atom, the proton number also determines the electron configuration, which influences chemical behaviour.
Special blocks of the Periodic Table
- s-block: Groups 1 and 2 plus helium; outer electrons in an s-subshell.
- p-block: Groups 13 to 18; outer electrons in a p-subshell.
- d-block (transition metals): Groups 3 to 12; outer electrons in a d-subshell.
- f-block (lanthanoids and actinoids): Inner transition metals, shown separately at the bottom.
Example
Explain why elements in the same group have similar chemical properties.
▶️Answer/Explanation
They have the same number of outer electrons, which determines their chemical reactions and bonding behaviour.
Example
Identify the period and group of an element with proton number 17.
▶️Answer/Explanation
Proton number 17 corresponds to chlorine.
Electron configuration: 2,8,7 — three shells means Period 3, and seven outer electrons means Group 17.
Example
How did Mendeleev’s arrangement differ from the modern Periodic Table?
▶️Answer/Explanation
Mendeleev arranged elements by increasing atomic mass and left gaps for undiscovered elements.
The modern table uses proton number, which resolves inconsistencies present in the mass-based arrangement.
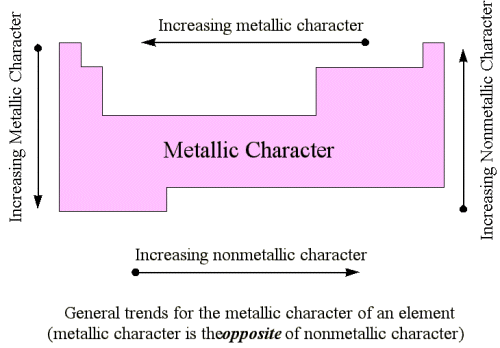
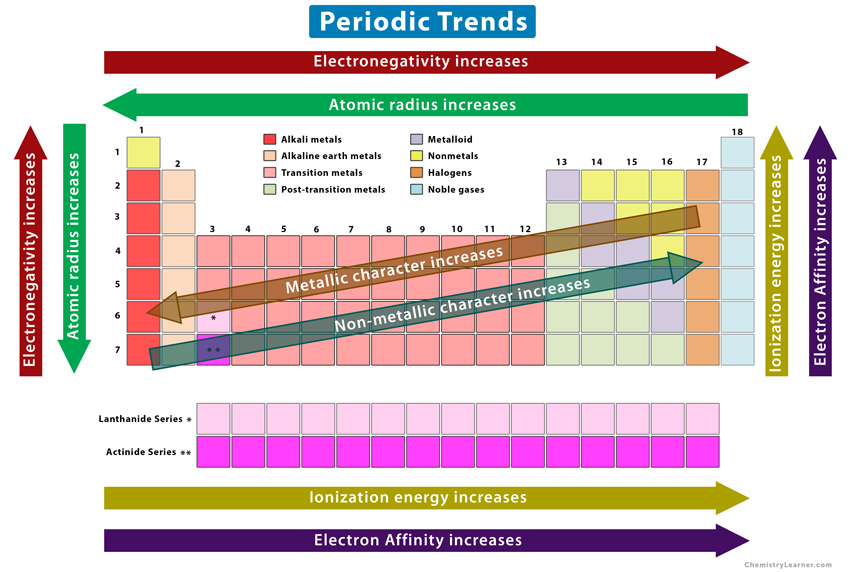
Change from metallic to non-metallic character across a period
Metallic and non-metallic character
- Metallic character is the tendency of an atom to lose electrons and form positive ions (cations).
- Non-metallic character is the tendency of an atom to gain electrons or share them in covalent bonds to form negative ions (anions) or molecules.

Trend across a period
- From left to right across a period, metallic character decreases while non-metallic character increases.
- Metals (Groups 1 and 2) are found on the left, metalloids in the centre, and non-metals (Groups 14–17) on the right.
- Group 18 elements (noble gases) are non-metals but largely inert due to full outer shells.
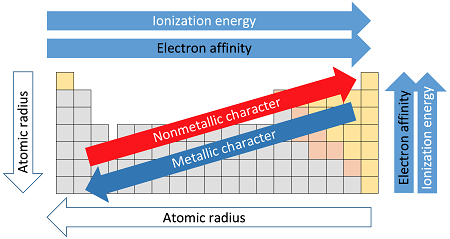
Reason for the trend
- Proton number increases across a period, increasing nuclear charge.
- Electrons are added to the same shell, so shielding remains similar.
- Stronger attraction between the nucleus and outer electrons makes it harder to lose electrons (reducing metallic character) and easier to gain electrons (increasing non-metallic character).
Example
Arrange the following Period 3 elements in order of decreasing metallic character: Na, Al, Mg, P, Cl.
▶️Answer/Explanation
Order: Na > Mg > Al > P > Cl.
Metallic character decreases across the period; sodium is most metallic, chlorine is strongly non-metallic.
Example
Explain why silicon is considered a metalloid.
▶️Answer/Explanation
Silicon shows metallic properties (can conduct electricity when doped) and non-metallic properties (forms covalent compounds).
Its position between metals and non-metals in the Periodic Table reflects this.
Example
Compare the ability of sodium and chlorine to form ions, linking to metallic and non-metallic character.
▶️Answer/Explanation
Sodium loses its outer electron easily to form \( \text{Na}^+ \), showing strong metallic character.
Chlorine gains an electron easily to form \( \text{Cl}^- \), showing strong non-metallic character.
Relationship between group number and the charge of ions formed
Relationship between group number and the charge of ions formed
General rule
- For main-group elements (Groups 1, 2, 13–18), the Group number indicates the number of electrons in the outer shell.
- Elements tend to form ions by losing or gaining electrons to achieve a stable noble gas configuration.
- Group number is directly linked to the charge of the ion formed.

Metallic groups (1, 2, 13)
- Metals lose all outer-shell electrons.
- Charge on ion = group number (positive sign).
- Examples:
- Group 1 → 1⁺ ions (Na⁺, K⁺)
- Group 2 → 2⁺ ions (Mg²⁺, Ca²⁺)
- Group 13 → 3⁺ ions (Al³⁺)
Non-metallic groups (15, 16, 17)
- Non-metals gain electrons to complete the outer shell.
- Charge on ion = (8 – group number) (negative sign).
- Examples:
- Group 17 → 1⁻ ions (Cl⁻, Br⁻)
- Group 16 → 2⁻ ions (O²⁻, S²⁻)
- Group 15 → 3⁻ ions (N³⁻, P³⁻)
Example
Predict the ion formed by a Group 2 element with proton number 20.
▶️Answer/Explanation
Proton number 20 = calcium.
Group 2 → 2 electrons in outer shell → loses both → \( \text{Ca}^{2+} \) ion formed.
Example
Explain why oxygen forms a 2⁻ ion.
▶️Answer/Explanation
Oxygen is in Group 16 → 6 outer electrons → needs 2 more to reach a stable octet → gains 2 electrons → \( \text{O}^{2-} \).
Example
Why doesn’t the Group number rule work for iron?
▶️Answer/Explanation
Iron is a transition metal (Group 8 in modern numbering) → can lose different numbers of electrons → forms \( \text{Fe}^{2+}\) and \(\text{Fe}^{3+}\) ions.
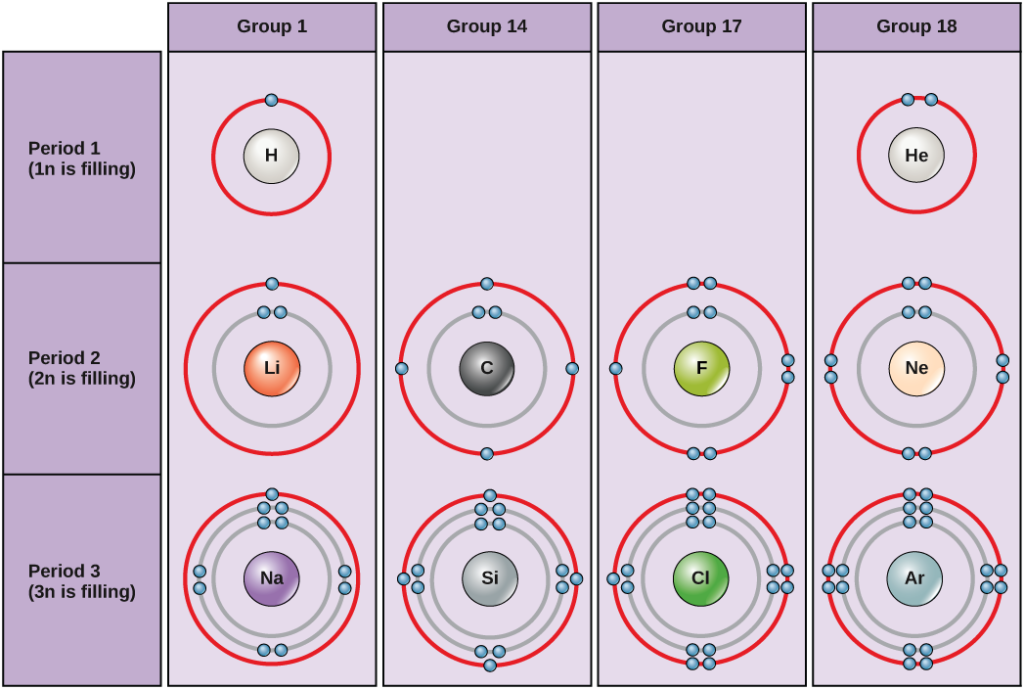
Similarities in chemical properties of elements in the same group
Similarities in chemical properties of elements in the same group
- Elements in the same group have the same number of electrons in their outermost (valence) shell.
- This leads to similar chemical properties because chemical reactions mainly involve the gain, loss, or sharing of these outer-shell electrons.

- The outer-shell configuration determines:
- The type of ions formed (positive or negative, and their charge)
- The type of bonds formed (ionic or covalent)
- Reactivity trends within the group
Why the properties are similar
- Although elements down a group have more electron shells (higher principal quantum number), the valence electron pattern is identical.
- Same valence electron pattern → same general reactivity pattern, even if speed of reaction changes.
- Differences in reactivity are due to changes in:
- Atomic radius (increases down a group)
- Electrostatic attraction between nucleus and outer electrons (decreases down a group)
- Ionisation energy (decreases down a group)
Example
Why do lithium, sodium, and potassium all react with water to form hydrogen gas and an alkali?
▶️Answer/Explanation
They are all in Group 1 → each has 1 outer electron → reacts by losing that electron to form an M⁺ ion → produces hydrogen gas and hydroxide ions in water.
Example
Explain why chlorine and bromine have similar chemical reactions with sodium.
▶️Answer/Explanation
Both are in Group 17 → 7 outer electrons → react by gaining 1 electron to form X⁻ ions → both form white crystalline sodium halide salts with sodium.
Predicting properties from the position of an element in the Periodic Table
Predicting properties from the position of an element in the Periodic Table
- The position of an element in the Periodic Table is determined by:
- Period number → tells you the number of electron shells.
- Group number (for main-group elements) → tells you the number of electrons in the outer shell.
- Block (s, p, d, f) → tells you the type of orbital where the last electron is found.

- This information allows you to predict:
- The type of bonding (metallic, ionic, covalent)
- The type and charge of ions formed
- Reactivity trends
- Physical properties (melting/boiling points, conductivity)
How position relates to properties
- Elements in the same group → similar chemical reactivity due to same outer electron configuration.
- Elements in the same period → gradual change in properties across the row, from metallic to non-metallic.
- From group number → deduce:
- Group 1 → lose 1 electron to form +1 ions.
- Group 2 → lose 2 electrons to form +2 ions.
- Group 17 → gain 1 electron to form -1 ions.
- From period number → deduce:
- Number of electron shells → affects size, ionisation energy, and reactivity trends.
Example
An element is in Group 2, Period 3. Predict its number of electron shells, number of valence electrons, and type of ion formed.
▶️Answer/Explanation
Group 2 → 2 valence electrons. Period 3 → 3 electron shells. Will lose 2 electrons to form a +2 ion (cation). Likely to be a reactive alkaline earth metal (e.g., magnesium).
Example
An element is in Group 17, Period 2. Predict its reactivity and type of bonding with sodium.
▶️Answer/Explanation
Group 17 → 7 valence electrons → needs 1 electron to complete outer shell → very reactive non-metal (halogen). Will gain 1 electron from sodium to form NaX (ionic bond), producing a white crystalline salt.
Example
How can the position of silicon (Group 14, Period 3) be used to predict its properties?
▶️Answer/Explanation
Group 14 → 4 valence electrons → can form covalent bonds by sharing electrons. Period 3 → 3 electron shells. Likely to have intermediate metallic/non-metallic properties → silicon is a metalloid with semiconductor behaviour.
Identify trends in groups from given element information
Identify trends in groups from given element information
- Elements in the same group show predictable trends in properties as you move down the group.
- Trends arise because elements in a group have the same number of valence electrons but increasing number of electron shells (period number).

Common trends in groups
- Atomic radius: increases down a group due to additional electron shells.
- Ionisation energy: decreases down a group as outer electrons are further from the nucleus and experience less attraction.
- Electronegativity: decreases down a group because atoms are larger and less able to attract bonding electrons.
- Reactivity:
- Metals (Groups 1 and 2): reactivity increases down the group.
- Non-metals (Groups 16 and 17): reactivity decreases down the group.
- Melting and boiling points: may increase or decrease depending on metallic or molecular structure.
Example
Given Li, Na, and K, identify the trend in reactivity with water.
▶️Answer/Explanation
All are Group 1 → 1 valence electron.
Reactivity increases down the group: K > Na > Li because the outer electron is further from nucleus and more easily lost.
Example
Given F, Cl, Br, predict the trend in reactivity.
▶️Answer/Explanation
All are Group 17 → 7 valence electrons.
Reactivity decreases down the group: F > Cl > Br because larger atoms attract electrons less strongly.
Example
Explain why ionisation energy decreases down Group 2.
▶️Answer/Explanation
Group 2 elements have the same number of valence electrons but increasing number of electron shells down the group → outer electrons are further from nucleus → less tightly held → lower ionisation energy.