Atomic structure and the Periodic Table - CIE iGCSE Chemistry Notes - New Syllabus
Atomic structure and the Periodic Table for iGCSE Chemistry Notes
Core Syllabus
- Describe the structure of the atom as a central nucleus containing neutrons and protons surrounded by electrons in shells
- State the relative charges and relative masses of a proton, a neutron and an electron
- Define proton number/atomic number as the number of protons in the nucleus of an atom
- Define mass number/nucleon number as the total number of protons and neutrons in the nucleus of an atom
- Determine the electronic configuration of elements and their ions with proton number 1 to 20, e.g. 2,8,3
- State that:
(a) Group VIII noble gases have a full outer electron shell
(b) the number of outer shell electrons is equal to the group number in Groups I to VII
(c) the number of occupied electron shells is equal to the period number
Atom and Subatomic particles
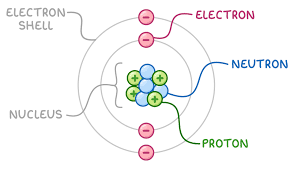
Structure of the Atom
An atom is the basic unit of matter. All substances are made up of atoms. Each atom has a very small, dense central region called the nucleus, which is surrounded by rapidly moving electrons in fixed energy levels called shells.
- Nucleus:
- Contains protons (positively charged particles)
- Contains neutrons (neutral particles with no charge)
- Is very small but contains almost all of the atom’s mass
- Electron Shells:
- Contain electrons (negatively charged particles)
- Electrons move very fast in specific shells or energy levels
- Electrons are arranged around the nucleus in these shells
- The shells fill in a fixed order: 2 in the first shell, 8 in the second, 8 in the third, etc.
Key Features:
- Atoms are electrically neutral overall: number of protons = number of electrons
- Mass of the atom is mainly due to the protons and neutrons (electrons have very small mass)
- The size of the nucleus is about 1/10,000 of the total diameter of the atom
Relative Charges and Masses of Subatomic Particles
Atoms are made up of three main types of subatomic particles: protons, neutrons, and electrons. These particles differ in charge and mass, and each has a specific role in the atom.
| Subatomic Particle | Relative Charge | Relative Mass | Location in Atom |
|---|---|---|---|
| Proton | +1 | 1 | Nucleus |
| Neutron | 0 (neutral) | 1 | Nucleus |
| Electron | -1 | 1/1836 (very small) | Electron shells (outside nucleus) |
Explanation:
- Protons are positively charged and give the atom its identity (e.g., 1 proton = hydrogen).
- Neutrons have no charge. They add to the mass of the atom and help stabilize the nucleus.
- Electrons are negatively charged and are found in energy levels (shells) around the nucleus. They are involved in chemical bonding and reactions.
Important Notes:
- Since electrons have such a small mass, their mass is often taken as zero when calculating atomic mass.
- In a neutral atom, the number of protons equals the number of electrons, so the total charge is zero.
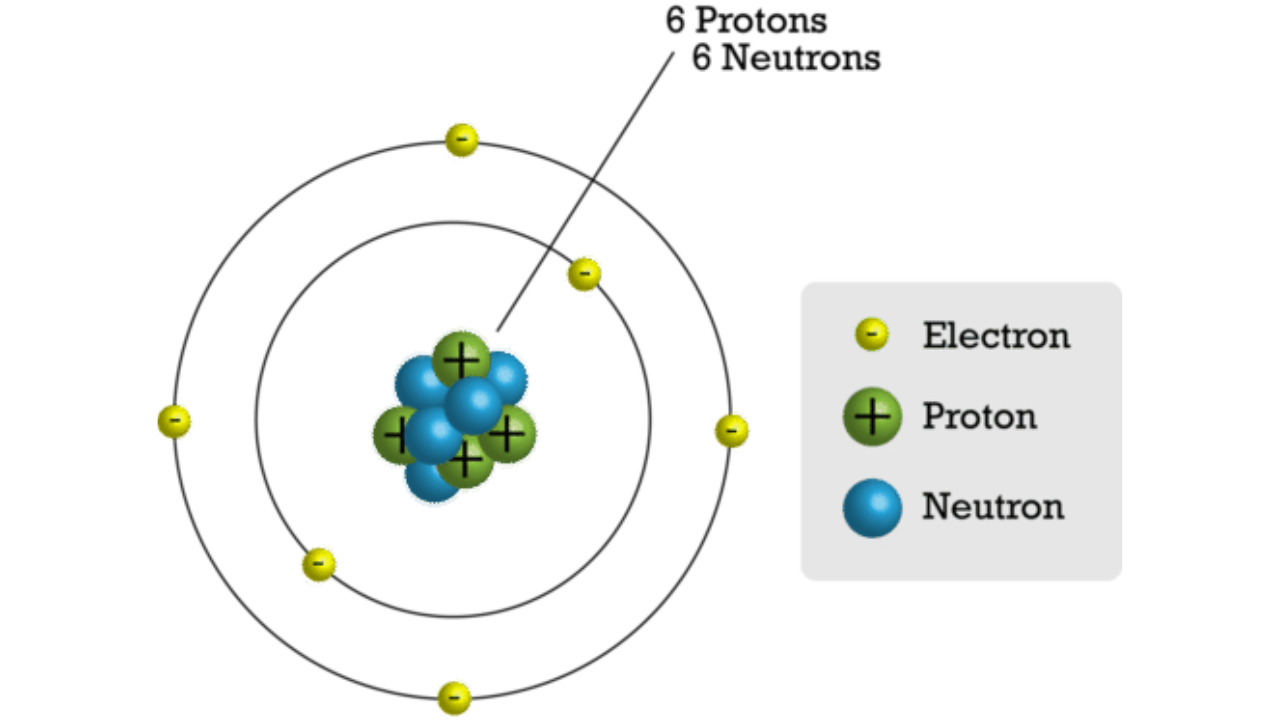
Example
An atom of carbon has 6 protons, 6 neutrons, and 6 electrons. Describe the structure of the carbon atom and state the relative charge and mass of each type of subatomic particle present.
▶️Answer/Explanation
- Structure of the atom: The carbon atom has a small central nucleus that contains 6 protons and 6 neutrons. Around this nucleus, 6 electrons are arranged in energy levels (shells).
- Subatomic particles:
- Protons: Relative charge = +1, Relative mass = 1
- Neutrons: Relative charge = 0, Relative mass = 1
- Electrons: Relative charge = -1, Relative mass ≈ 1/1836 (very small)
- Summary: The atom is overall neutral because the number of positively charged protons equals the number of negatively charged electrons.
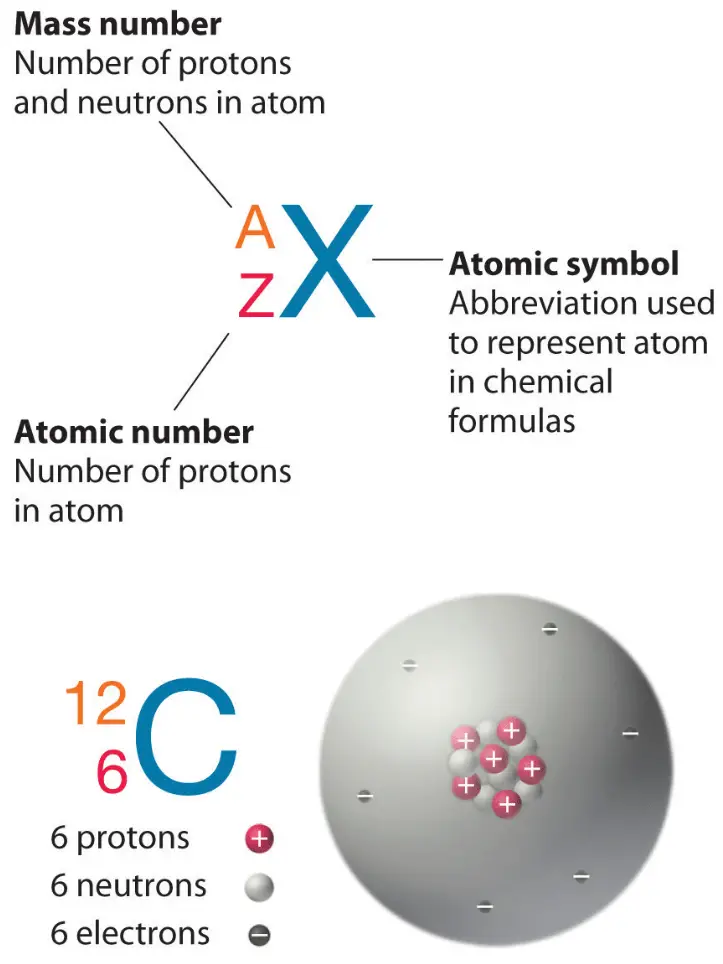
Atomic number and Mass number
Proton Number / Atomic Number
The proton number, also known as the atomic number, is defined as the number of protons in the nucleus of an atom.
This number is unique for every element and determines the identity of the element. For example:
- Hydrogen has 1 proton → Atomic number = 1
- Carbon has 6 protons → Atomic number = 6
- Oxygen has 8 protons → Atomic number = 8
Key Facts:
- All atoms of the same element have the same number of protons.
- The proton number also tells us how many electrons a neutral atom has (because in a neutral atom: number of protons = number of electrons).
- Elements in the Periodic Table are arranged in order of increasing proton number.
Example:
Sodium (Na) has a proton number of 11. This means every atom of sodium has:
- 11 protons in its nucleus
- 11 electrons (in a neutral atom)
Mass Number / Nucleon Number
The mass number, also called the nucleon number, is defined as the total number of protons and neutrons in the nucleus of an atom.
Electrons are not included in this number because their mass is negligible compared to that of protons and neutrons.
Formula:
Mass number = Number of protons + Number of neutrons

Key Facts:
- The mass number gives the approximate mass of an atom since most of the atom’s mass is in the nucleus.
- Atoms of the same element can have different mass numbers. These are called isotopes.
- The number of neutrons in an atom can be found using:
Number of neutrons = Mass number – Proton number
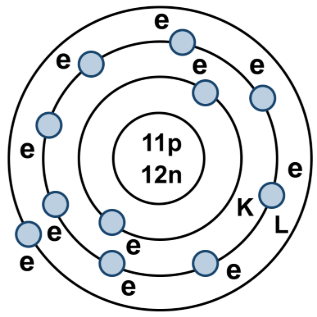
Example
An atom of sodium has 11 protons and 12 neutrons. What are the proton number and mass number of this sodium atom?
▶️Answer/Explanation
- Proton number (atomic number): The number of protons = 11
- Mass number (nucleon number): Total number of protons + neutrons = 11 + 12 = 23
- Therefore:
- Proton number = 11
- Mass number = 23
The sodium atom can be represented as:
\( ^{23}_{11}Na \)
Electronic Configuration
Electronic Configuration
Electronic configuration refers to the arrangement of electrons in an atom’s electron shells (also called energy levels).
Electrons are arranged around the nucleus in specific shells. Each shell has a maximum number of electrons it can hold:
- First shell: up to 2 electrons
- Second shell: up to 8 electrons
- Third shell: up to 8 electrons (for the first 20 elements)
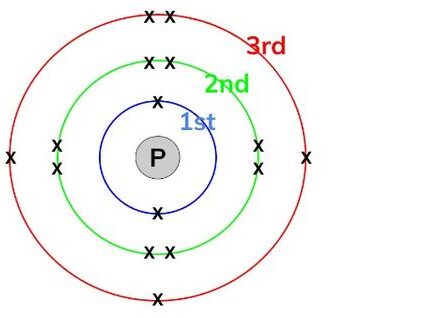
The number of electrons in an atom equals the number of protons (proton number or atomic number), since atoms are neutral.
Electronic configuration is written using numbers to show how many electrons are in each shell, separated by commas. For example: 2,8,1
Examples of elements (atoms):
- Hydrogen (H), proton number = 1 → electronic configuration: 1
- Carbon (C), proton number = 6 → 2,4
- Oxygen (O), proton number = 8 → 2,6
- Sodium (Na), proton number = 11 → 2,8,1
- Calcium (Ca), proton number = 20 → 2,8,8,2
Electronic Configuration of Ions:
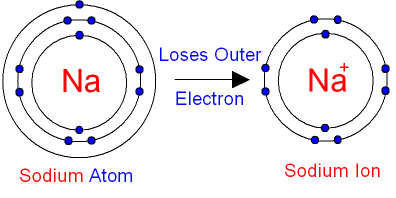
- Positive ions (cations): formed when atoms lose electrons. The electronic configuration changes because there are fewer electrons.
- Negative ions (anions): formed when atoms gain electrons.
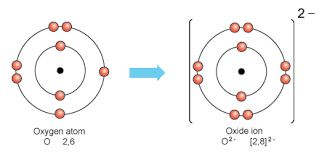
Examples of ions:
- Na (2,8,1) → Na⁺ loses 1 electron → 2,8
- Mg (2,8,2) → Mg²⁺ loses 2 electrons → 2,8
- Cl (2,8,7) → Cl⁻ gains 1 electron → 2,8,8
- O (2,6) → O²⁻ gains 2 electrons → 2,8
Group and Period Patterns from Electronic Configuration
The Periodic Table is arranged in a way that reflects the electronic configurations of elements. It helps predict chemical properties and how elements react.
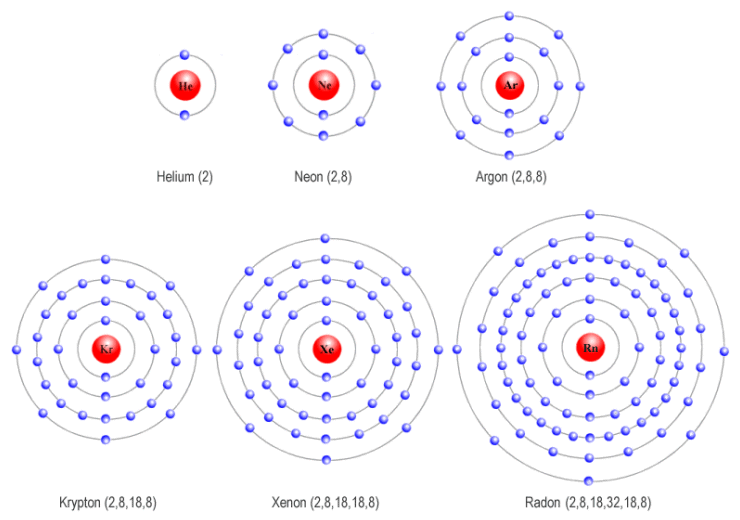
(a) Group VIII noble gases have a full outer electron shell
- Group VIII (also called Group 0) elements are the noble gases: He, Ne, Ar, Kr, etc.
- All noble gases have full outer shells, making them very stable and unreactive.
- Examples:
- Helium (He): 2 electrons → full first shell → stable
- Neon (Ne): 2,8 → full second shell → stable
- Argon (Ar): 2,8,8 → full third shell → stable
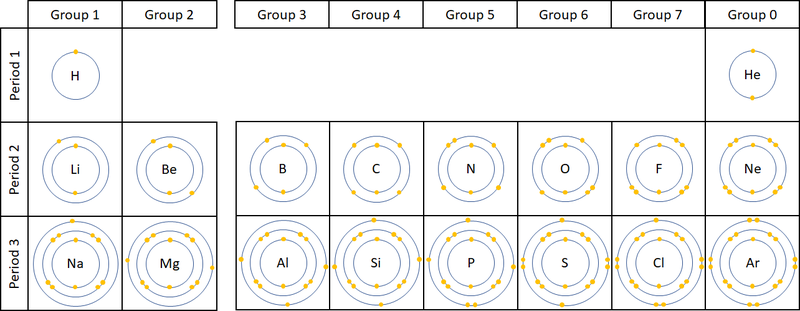
(b) The number of outer shell electrons = group number (for Groups I to VII)
- For elements in Groups I to VII, the group number equals the number of electrons in the outermost shell.
- This pattern only applies to main group elements (not transition metals).
- Examples:
- Group I – Lithium (Li): 2,1 → 1 outer electron
- Group II – Magnesium (Mg): 2,8,2 → 2 outer electrons
- Group VII – Chlorine (Cl): 2,8,7 → 7 outer electrons
(c) The number of occupied electron shells = period number
- The period number tells how many electron shells an atom of an element uses.
- Examples:
- Hydrogen (H): 1 shell → Period 1
- Beryllium (Be): 2,2 → 2 shells → Period 2
- Sodium (Na): 2,8,1 → 3 shells → Period 3
- Calcium (Ca): 2,8,8,2 → 4 shells → Period 4
Example
Phosphorus has a proton number of 15. What is the electronic configuration of phosphorus? Based on this, determine its group and period in the Periodic Table.
▶️Answer/Explanation
- Total electrons = 15 (since phosphorus is neutral)
- Step 1 – Electronic configuration: Distribute the electrons into shells:
2 electrons in the first shell
8 electrons in the second shell
5 electrons in the third shell
Configuration = 2,8,5 - Step 2 – Determine the Period: The number of shells = 3 → So phosphorus is in Period 3
- Step 3 – Determine the Group: The number of outer shell electrons = 5 → So phosphorus is in Group V
Final Answer: Phosphorus has the electronic configuration 2,8,5. It belongs to Group V and Period 3 of the Periodic Table.
