Carboxylic acids- CIE iGCSE Chemistry Notes - New Syllabus
Carboxylic acids for iGCSE Chemistry Notes
Core Syllabus
- Describe the reaction of ethanoic acid with:
(a) metals
(b) bases
(c) carbonates
including names and formulae of the salts produced
Supplement Syllabus
- Describe the formation of ethanoic acid by the oxidation of ethanol:
(a) with acidified aqueous potassium manganate(VII)
(b) by bacterial oxidation during vinegar production - Describe the reaction of a carboxylic acid with an alcohol using an acid catalyst to form an ester
Reactions of Ethanoic Acid
Reactions of Ethanoic Acid
Ethanoic acid (\( \text{CH}_3\text{COOH} \)) is a weak organic acid.Like other acids, it reacts with metals, bases, and carbonates to form characteristic products.
(a) Reaction with Metals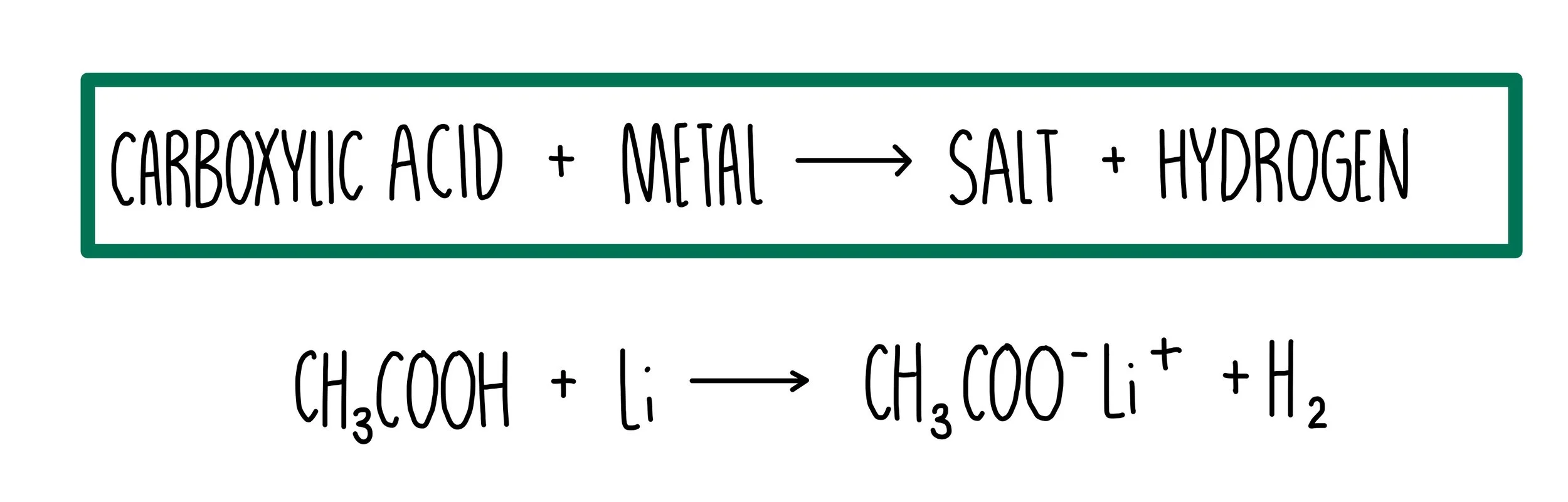
Ethanoic acid reacts with reactive metals such as magnesium and zinc to produce a salt (metal ethanoate) and hydrogen gas.
Example:
\( 2\text{CH}_3\text{COOH} + \text{Mg} \rightarrow (\text{CH}_3\text{COO})_2\text{Mg} + \text{H}_2 \)
In this case, the salt is magnesium ethanoate.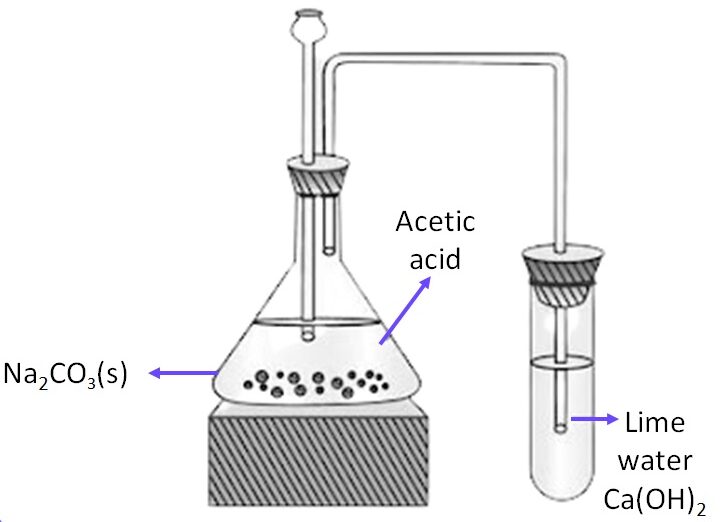
(b) Reaction with Bases
Ethanoic acid reacts with metal hydroxides to form a salt (metal ethanoate) and water.
Example:
\( \text{CH}_3\text{COOH} + \text{NaOH} \rightarrow \text{CH}_3\text{COONa} + \text{H}_2\text{O} \)
The salt formed here is sodium ethanoate.
(c) Reaction with Carbonates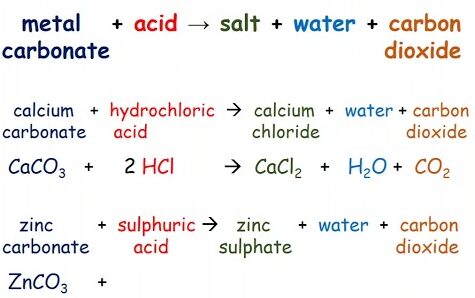
Ethanoic acid reacts with metal carbonates to produce a salt (metal ethanoate), carbon dioxide, and water.
Example:
\( 2\text{CH}_3\text{COOH} + \text{Na}_2\text{CO}_3 \rightarrow 2\text{CH}_3\text{COONa} + \text{H}_2\text{O} + \text{CO}_2 \)
The effervescence of carbon dioxide gas is observed in this reaction.
Example
Write equations for the reactions of ethanoic acid with magnesium, sodium hydroxide, and calcium carbonate. Name the salts formed.
▶️Answer/Explanation
(a) Reaction with a metal (magnesium):
\( 2\text{CH}_3\text{COOH} + \text{Mg} \rightarrow (\text{CH}_3\text{COO})_2\text{Mg} + \text{H}_2 \)
Salt formed: Magnesium ethanoate
(b) Reaction with a base (sodium hydroxide):
\( \text{CH}_3\text{COOH} + \text{NaOH} \rightarrow \text{CH}_3\text{COONa} + \text{H}_2\text{O} \)
Salt formed: Sodium ethanoate
(c) Reaction with a carbonate (calcium carbonate):
\( 2\text{CH}_3\text{COOH} + \text{CaCO}_3 \rightarrow (\text{CH}_3\text{COO})_2\text{Ca} + \text{H}_2\text{O} + \text{CO}_2 \)
Salt formed: Calcium ethanoate
Formation of Ethanoic Acid by Oxidation of Ethanol
Formation of Ethanoic Acid by Oxidation of Ethanol
Ethanoic acid (\( \text{CH}_3\text{COOH} \)) can be produced by oxidising ethanol (\( \text{CH}_3\text{CH}_2\text{OH} \)). This can happen chemically using oxidising agents or naturally by bacterial action.
(a) Oxidation with Acidified Potassium Manganate(VII)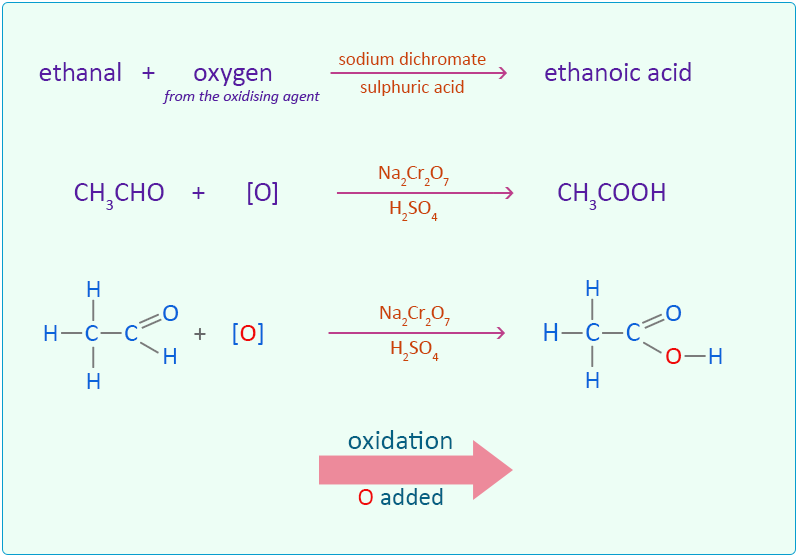
Ethanol is oxidised to ethanoic acid when treated with potassium manganate(VII) (\( \text{KMnO}_4 \)) in acidic conditions.
The purple colour of \( \text{MnO}_4^- \) ions changes to colourless as it is reduced.
Equation:
\( \text{CH}_3\text{CH}_2\text{OH} + [O] \rightarrow \text{CH}_3\text{COOH} + \text{H}_2\text{O} \)
This is a common laboratory method of preparing ethanoic acid.
(b) Bacterial Oxidation (Vinegar Production)
In the presence of oxygen, bacteria such as Acetobacter oxidise ethanol to ethanoic acid. This process occurs naturally when alcoholic drinks like wine are left exposed to air, producing vinegar (a dilute solution of ethanoic acid).
Equation:
\( \text{CH}_3\text{CH}_2\text{OH} + \text{O}_2 \rightarrow \text{CH}_3\text{COOH} + \text{H}_2\text{O} \)
Reaction of a Carboxylic Acid with an Alcohol to Form an Ester
Reaction of a Carboxylic Acid with an Alcohol to Form an Ester
Carboxylic acids react with alcohols in the presence of an acid catalyst to produce esters. This reaction is called esterification.
Reaction conditions:
- Carboxylic acid (e.g. ethanoic acid)
- Alcohol (e.g. ethanol)
- Concentrated sulfuric acid (\( \text{H}_2\text{SO}_4 \)) as the catalyst
- Heat
Word equation: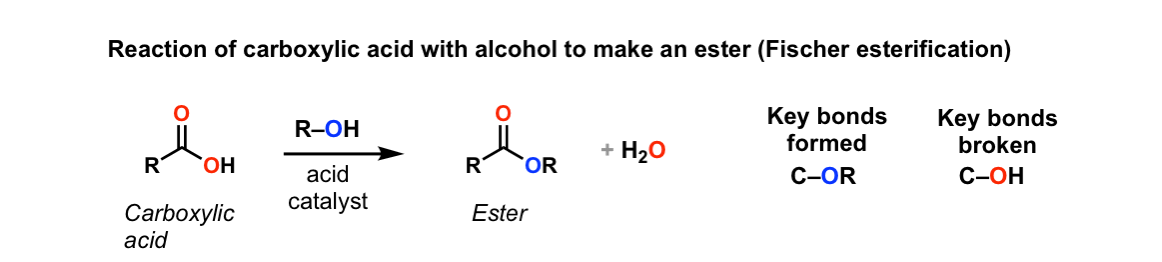
ethanoic acid + ethanol → ethyl ethanoate + water
Chemical equation:
\( \text{CH}_3\text{COOH} + \text{CH}_3\text{CH}_2\text{OH} \rightarrow \text{CH}_3\text{COOCH}_2\text{CH}_3 + \text{H}_2\text{O} \)
Explanation:
- The –OH group from the carboxylic acid and a hydrogen atom from the alcohol combine to form water.
- The remaining parts join together to form the ester.
Properties of Esters:
- Pleasant, fruity smell
- Used in flavourings and perfumes
- Volatile compared to carboxylic acids
Example:
Ethanoic acid + ethanol → ethyl ethanoate (pleasant fruity ester) + water
Example
Write the equation for the esterification of ethanoic acid with 2-methylpropan-1-ol. Name the ester formed.
▶️Answer/Explanation
Reaction type: Esterification.
- Reactants: ethanoic acid (\( \text{CH}_3\text{COOH} \)) and 2-methylpropan-1-ol (\( \text{CH}_3\text{-CH}(\text{CH}_3)\text{-CH}_2\text{OH} \)).
- Catalyst: concentrated \( \text{H}_2\text{SO}_4 \).
- Equation:
\( \text{CH}_3\text{COOH} + \text{CH}_3\text{-CH}(\text{CH}_3)\text{-CH}_2\text{OH} \rightarrow \text{CH}_3\text{COOCH}_2\text{-CH}(\text{CH}_3)\text{-CH}_3 + \text{H}_2\text{O} \). - Product: 2-methylpropyl ethanoate and water.
- Observation: ester has a sweet/fruity smell.
